13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racem..
$ 9.99 · 4.6 (722) · In stock

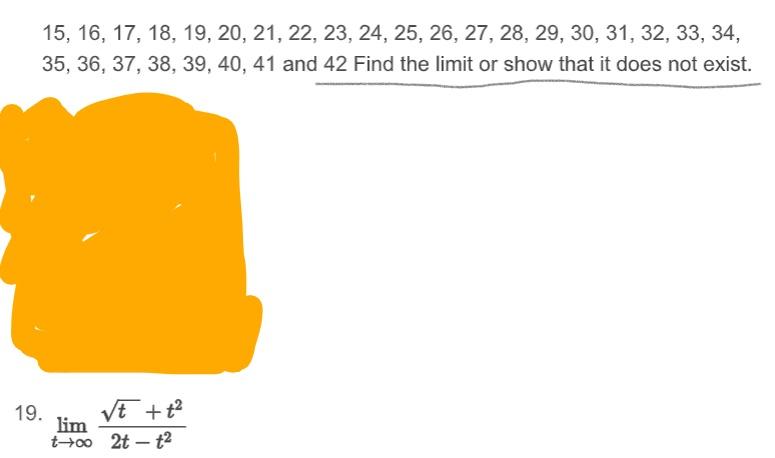
Solution For 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g)
13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is:
Video solution 1: 13 14 15 16 17 18 19 20 21 22 23 2425 26 27 28 29 30 31 32 40% of a racemic mixture of 0.2 moles of N2 and 0.6 moles of H2 react to give NH3 according to the equation, N2( g)+H2( g) ₹ pressure. Then the ratio of the final volume to the initial volume of gases is

Continued 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
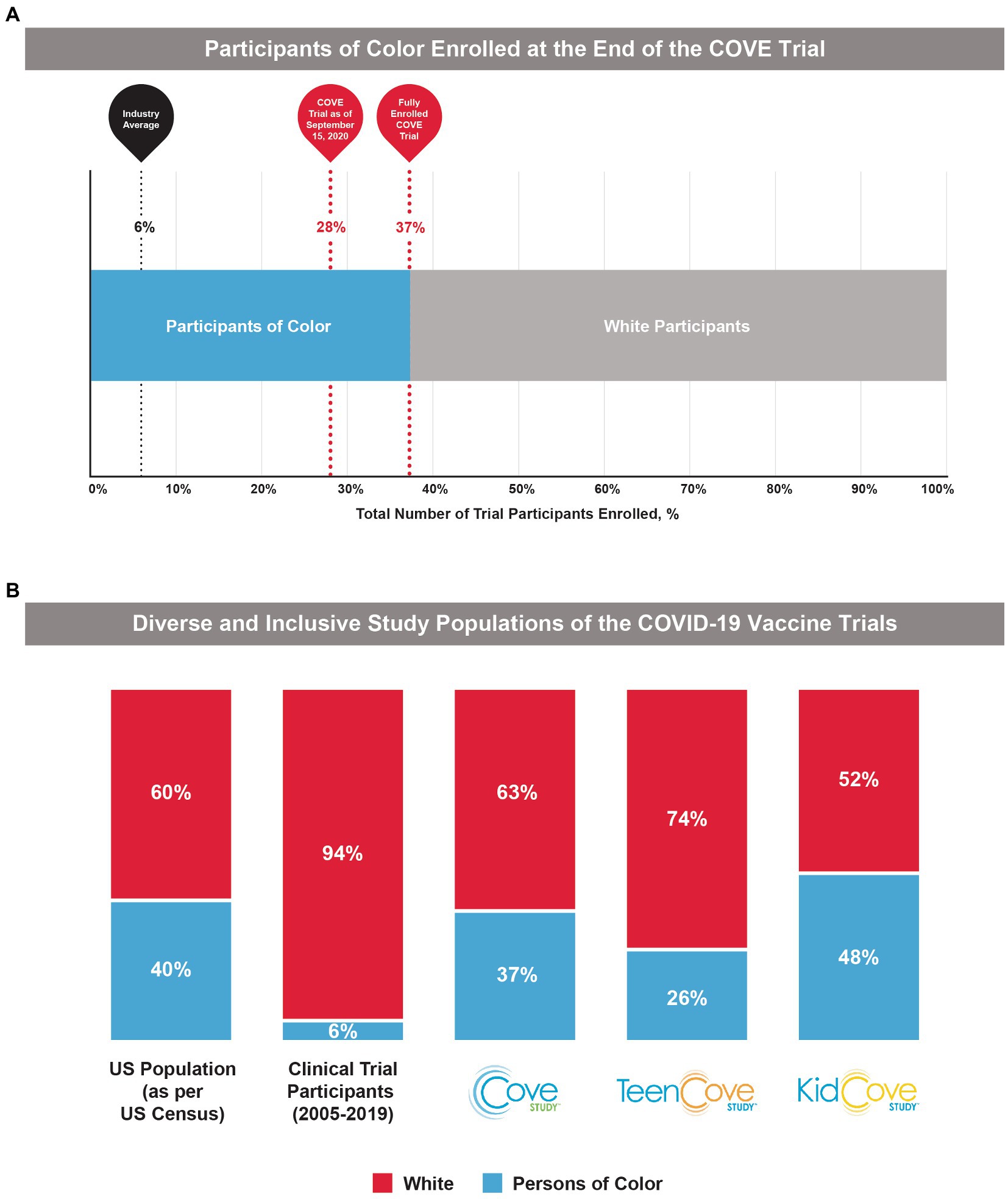
Frontiers Diversity and inclusion in clinical trials: Evolution
Solved 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27

1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
T. Rowe Price's 10th Annual Parents, Kids & Money Survey
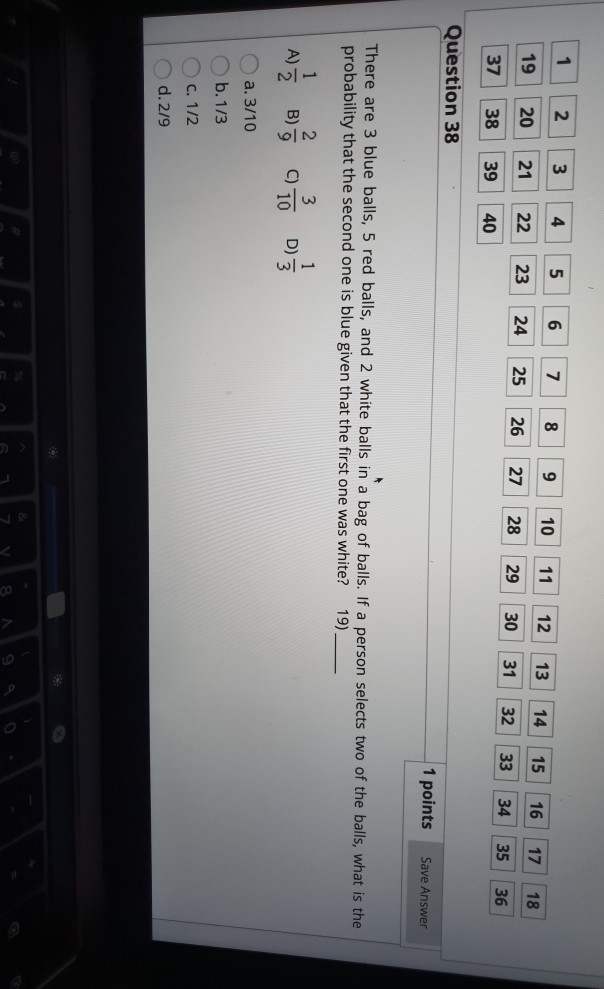
Solved 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 19 20 17 21 18
Nutrients, Free Full-Text

Socioeconomic variation in the financial consequences of ill

Cancers, Free Full-Text
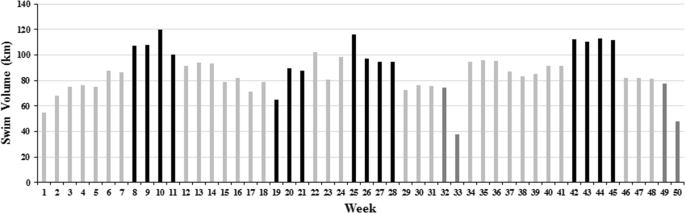
Contemporary Periodization of Altitude Training for Elite

Falcon Field Airport Master Plan by City of Mesa, AZ - Issuu
Solved 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21

image.isu.pub/220511013323-ec794add00003185489695f
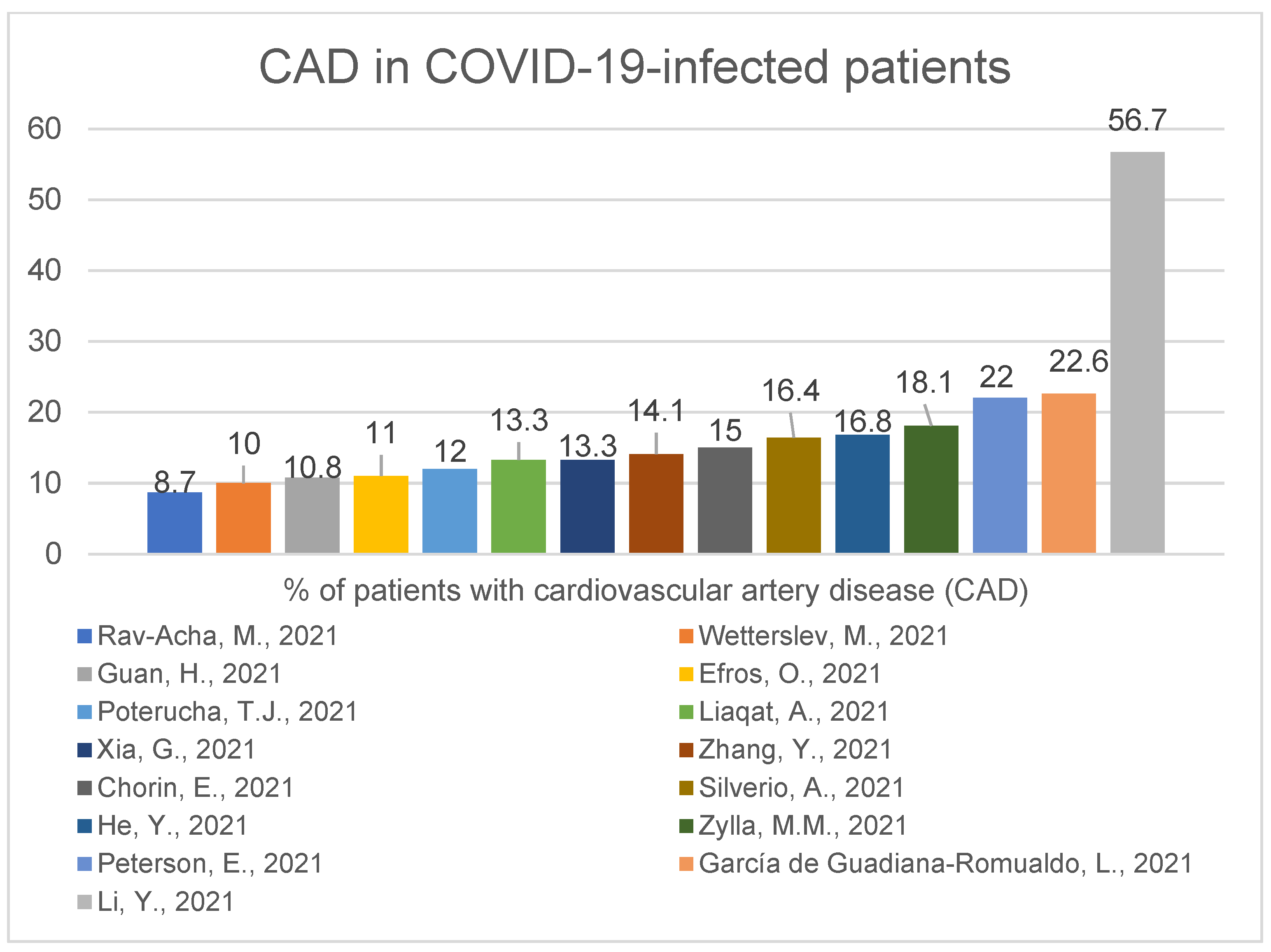
IJERPH, Free Full-Text
Prepping for the Glaucoma Grind