200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
$ 14.99 · 4.7 (569) · In stock
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Universal Kinetic Description for Thermal Decomposition of Copper(II) Hydroxide over Different Water Vapor Pressures

Climate-Driven Variations in the Depositional Environment and Organic Matter Accumulation of Lacustrine Mudstones: Evidence from Organic and Inorganic Geochemistry in the Biyang Depression, Nanxiang Basin, China
Basic Chemical Calculations-Merged, PDF, Mole (Unit)

0958 ch11.pdf - Index of - Free

new developments in the generation of controlled atmospheres
Xi To Xii A Step Forward To Iitjee, PDF, Redox
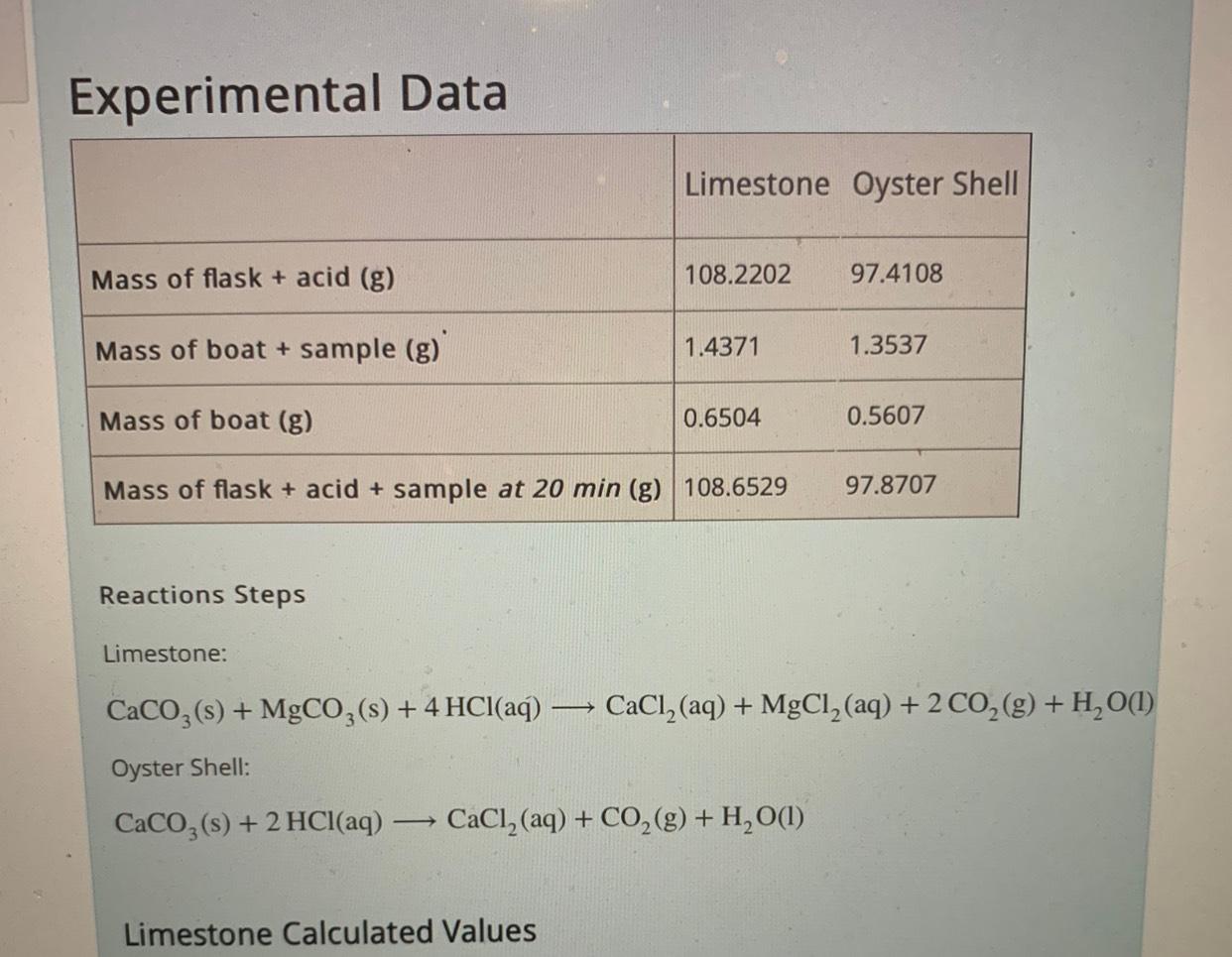
Solved A-What is the mass of CO2 lost at 20 min from the

6.5 g of an impure sample of limestone liberates 2.2 g of CO2 on strong heating. The percentage purity of

American Chemical Society - ACS: Division of Environmental

Wu unswer the questions given below it: 150 ml of N HCI is required to react completely with 1.0 g of a sample of limestone. Calculate the percentage purity of CaCO3. (A)

LITHIUM AMERICAS CORP. - Drilling Campaign - EX-99.1 - February 06, 2023
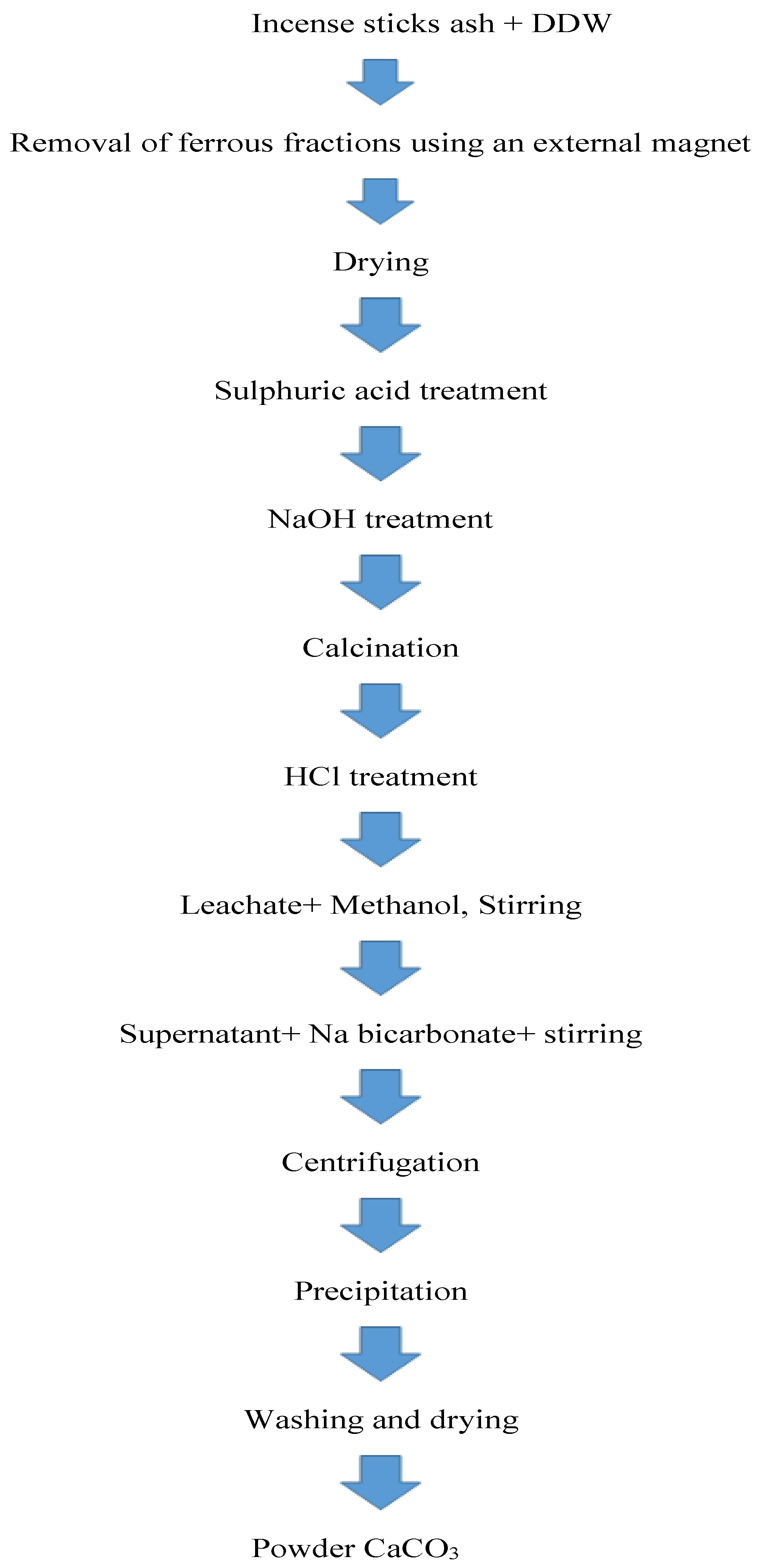
Applied Sciences, Free Full-Text