Compression Factor and Fugacity
$ 14.50 · 5 (135) · In stock

Share your videos with friends, family and the world

SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1
Molecules, Free Full-Text

CHEM 111 : Physical Chemistry - University of the Philippines Los Baños

DOC) Computation of the Compression Factor and Fugacity Coefficient of Real Gases
qph.cf2.quoracdn.net/main-qimg-dafd7e8fefa47c5b6c2
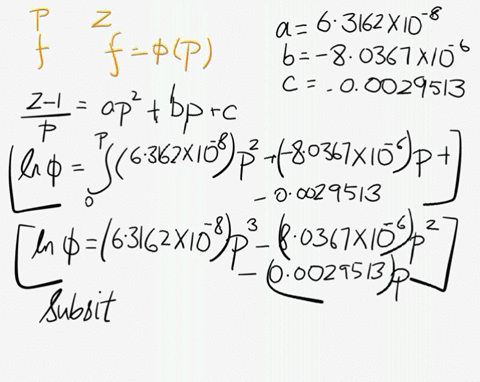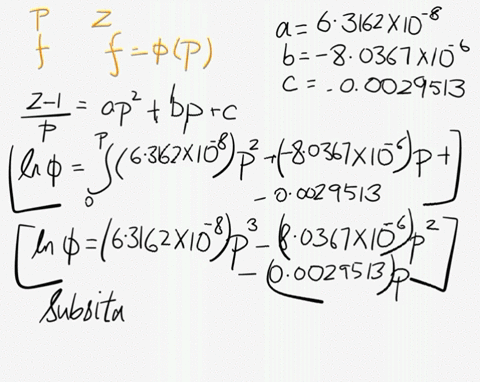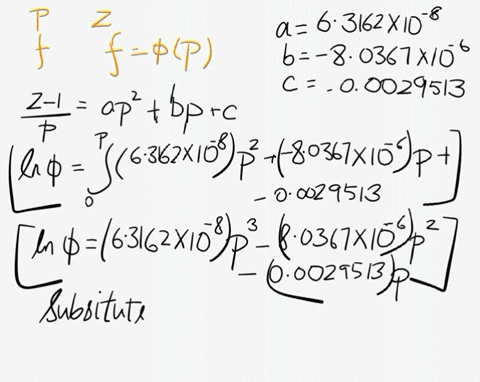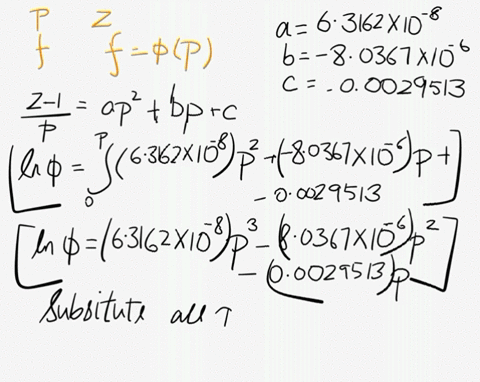
SOLVED: At 200 K, the compression factor of oxygen varies with pressure as shown below. Evaluate the fugacity of oxygen at this temperature and 100 atm: p (atm) Z 1,0000 0.9971 4.00000 0.98796 7,00000 0.97880 10,0000 0.96956 40.00 0.8734 70.00 0.7764

CHEM111.1 - Exer2 Full-Report.docx - Name: Nicole Ann M. Pedrina Date Performed: Aug. 30 & Sept. 6 '18 Group No.: 1 Date Submitted: Sept. 14 '18 Section

CHEM 111.1 : Physical Chemistry I (lab) - University of the Philippines Los Baños
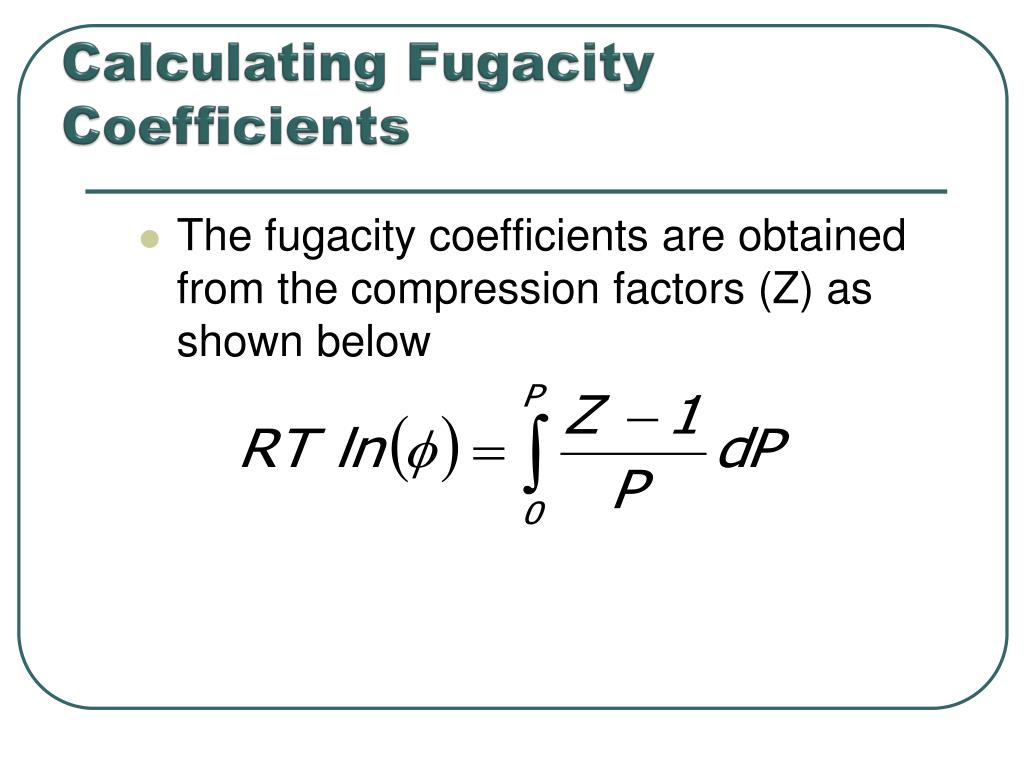
PPT - Chemistry 231 PowerPoint Presentation, free download - ID
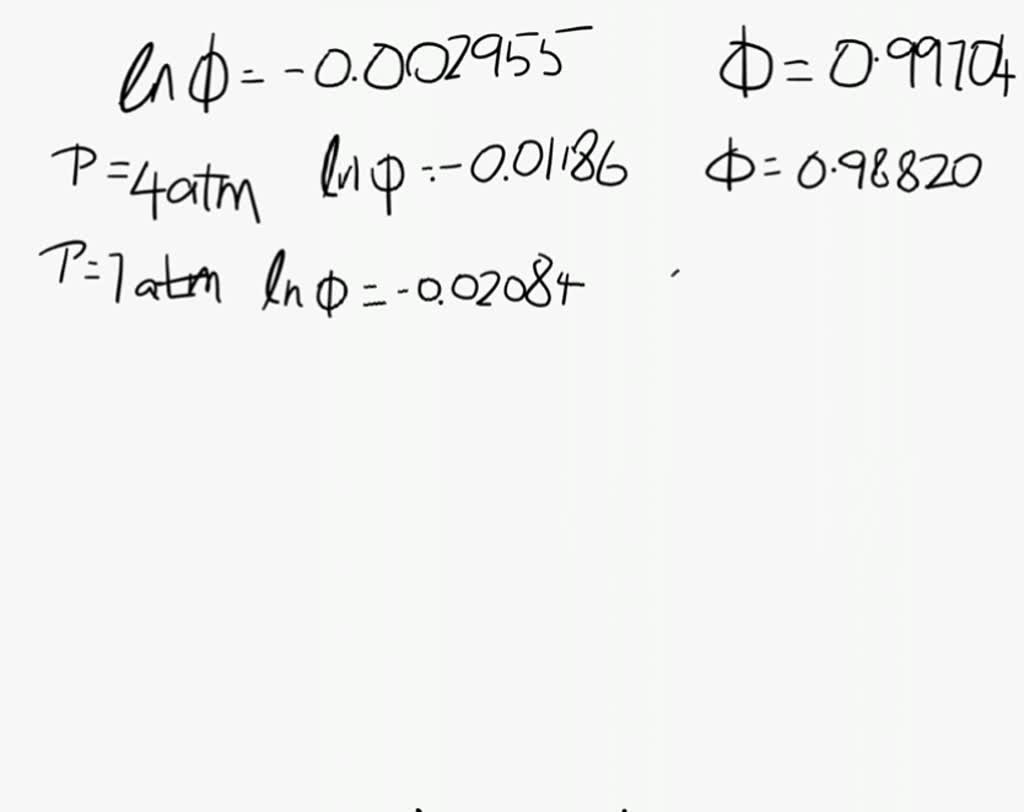
SOLVED: At 200 K, the compression factor of oxygen varies with pressure as shown below. Evaluate the fugacity of oxygen at this temperature and 100 atm: p (atm) Z 1,0000 0.9971 4.00000 0.98796 7,00000 0.97880 10,0000 0.96956 40.00 0.8734 70.00 0.7764
Why is fugacity, or z, always less than 1? - Quora

Compressibility factor - Wikipedia
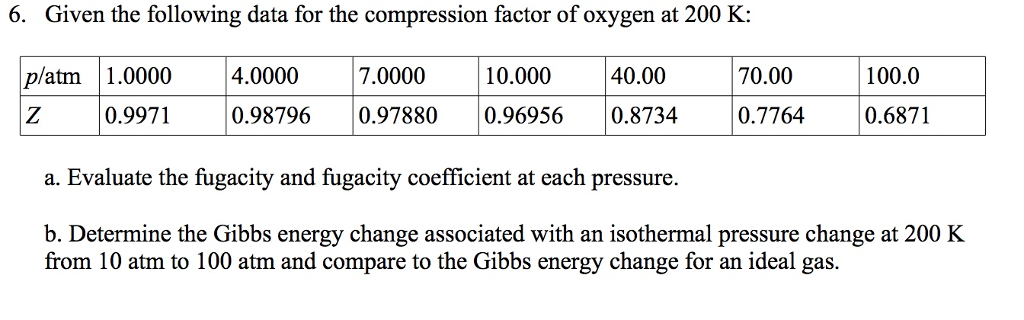
Solved 6. Given the following data for the compression