Continuously expandable interbody spacer in (A) minimized and (B)
$ 18.99 · 4.8 (86) · In stock

Download scientific diagram | Continuously expandable interbody spacer in (A) minimized and (B) expanded forms (RISE-L Globus Medical, Inc, Audubon, PA). from publication: Comparative Effectiveness of Expandable Versus Static Interbody Spacers via MIS LLIF: A 2-Year Radiographic and Clinical Outcomes Study | Study Design Retrospective cohort study. Objective The purpose of this study is to compare the radiographic and clinical outcomes of expandable interbody spacers to static interbody spacers. Methods This is a retrospective, institutional review board–exempt chart review of | Static, Mullerian Inhibiting Substance (MIS) and Outcome Assessment (Health Care) | ResearchGate, the professional network for scientists.

Transforaminal lumbar interbody fusion with an expandable interbody device: Two-year clinical and radiographic outcomes - North American Spine Society Journal (NASSJ)

Journal of Minimally Invasive Spine Surgery and Technique

Magnetic resonance imaging features of disc disease
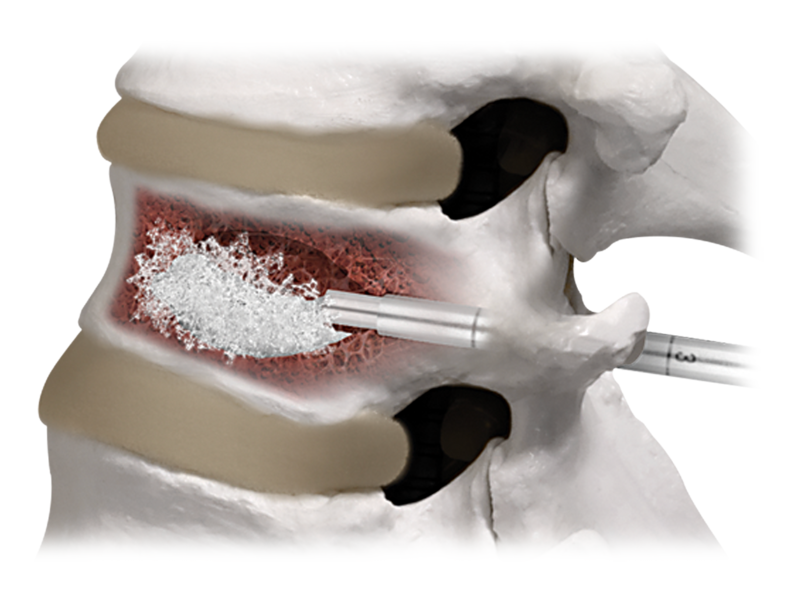
Spinal Products for Musculoskeletal Solutions

Percutaneous Endoscopic Cervical Diskectomy and Stabilization

Charles LEDONIO, Senior Director, Exactech, Inc., Florida, Clinical Affairs

Continuously expandable interbody spacer in (A) minimized and (B)
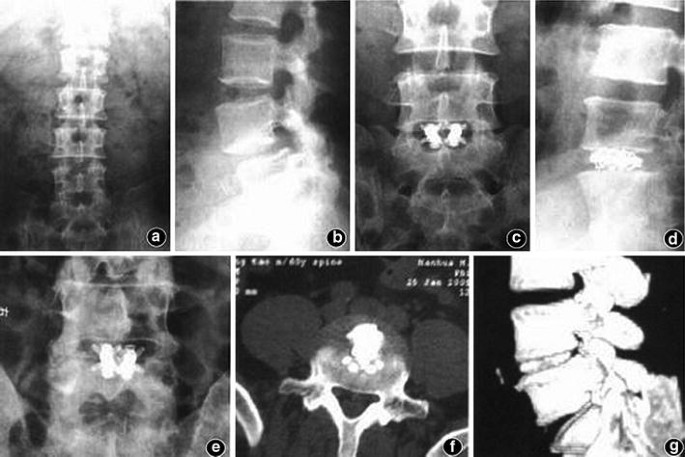
Percutaneous endoscopic lumbar discectomy and interbody fusion with B-Twin expandable spinal spacer

Expandable Polyaryl-Ether-Ether-Ketone Spacers for Interbody Distraction in the Lumbar Spine. - Abstract - Europe PMC

James Towner's research works University of Rochester, Rochester (UR) and other places

Yan Michael LI, MD PhD, University of Texas MD Anderson Cancer Center, Texas, MD Anderson
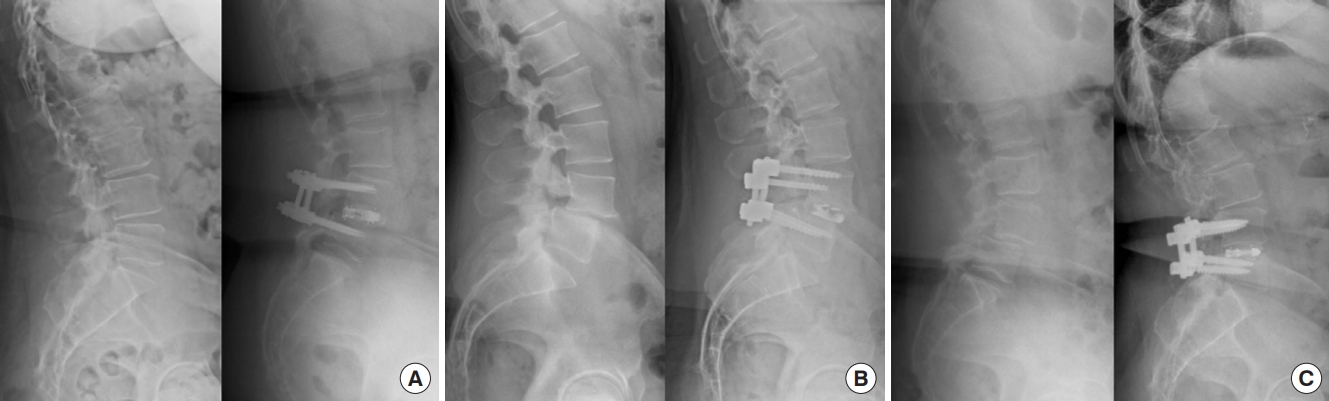
Early Experience With Uniplanar Versus Biplanar Expandable Interbody Fusion Devices in Single-Level Minimally Invasive Transforaminal Lumbar Interbody Fusion