Applications for Medical Device Investigational Testing Authorizations Guidance Document
$ 6.99 · 4.9 (67) · In stock

Applications for Medical Device Investigational Testing Authorizations Guidance Document

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

FDA Releases Guidance On Cybersecurity In Medical Devices
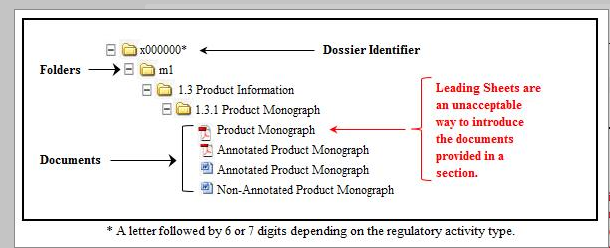
Guidance document: preparation of regulatory activities in non

2023 annual report from US FDA medical device regulatory division

Canadian Medical Device Regulations 101

Clinical Investigation - an overview

FDA Emergency Use Authorizations

Medical Device Guidelines and Regulations Handbook

Medical Device Regulations and Guidelines
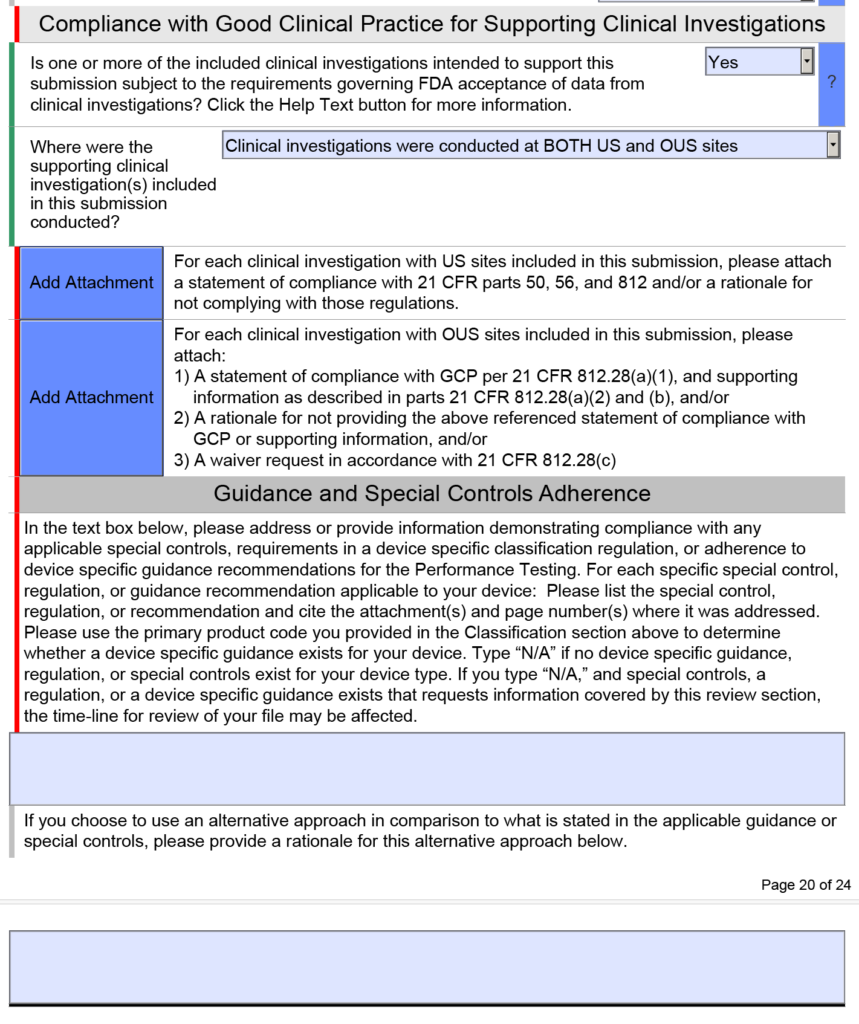
/wp-content/uploads/FDA-eS

Guidance Document for the Completion of APHIS/CDC Form 5