Hydrogen spectral series - Wikipedia
$ 20.00 · 4.6 (635) · In stock

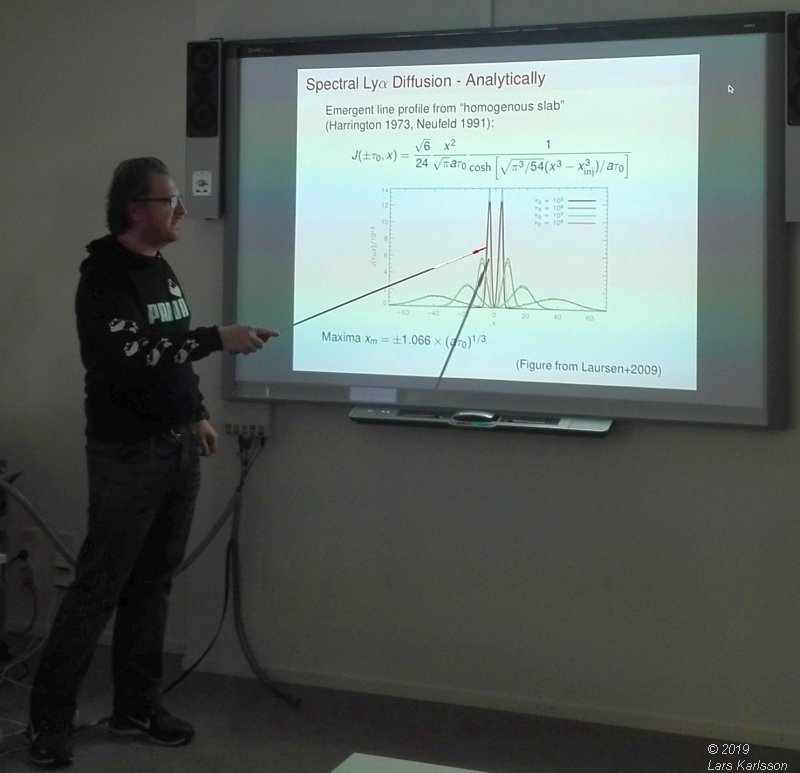
Lyman Spectra by Christian Herenz, 2019
How does the emitted spectra of an element depend on the structure of an atom of an element? - Quora
What is the wavelength of absorbtion for the individual elements? Is there anywhere which has a list? I have tried searching and appear to get varied answers. I would presume <200nm for
If the series limit of the Lyman series for a hydrogen atom is 912A°, what is the series limit of wavelength for the Balmer series for the hydrogen atom? - Quora
What is the calculated energy (in kJ) of light emitted in the hydrogen spectrum, when an electron falls from the 4th energy level to the 3rd energy level? - Quora

Quantum Mechanics in Three Dimensions
Mobile Workshop Supplies Pty Ltd Moonah TAS, 41% OFF

Difference Between Hydrogen and Helium Emission Spectra
The Need for Quantum Mechanics
What is the reason for spectral lines in the hydrogen spectrum? How do we explain it? - Quora
The frequency of one of the lines in the Paschen series of hydrogen atom is 2.33*10^14 hertz. What is the quantum number which causes this transition? - Quora

Why is the number of lines observed in the hydrogen spectrum very large? - Quora
If the series limit of the Lyman series for a hydrogen atom is 912A°, what is the series limit of wavelength for the Balmer series for the hydrogen atom? - Quora

