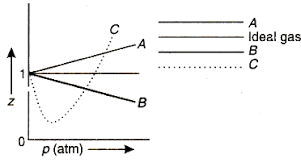
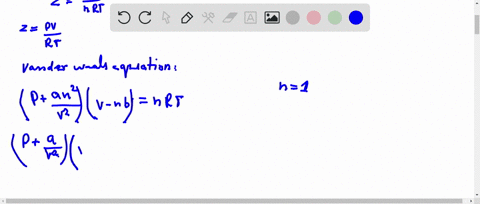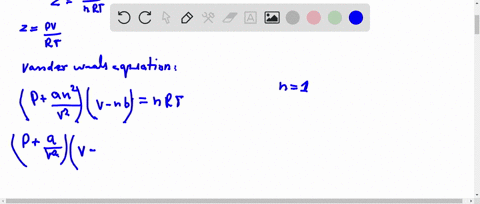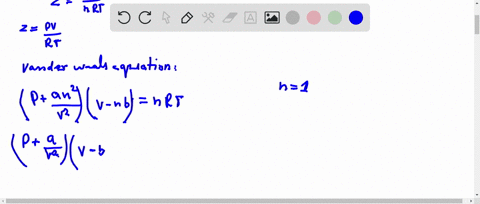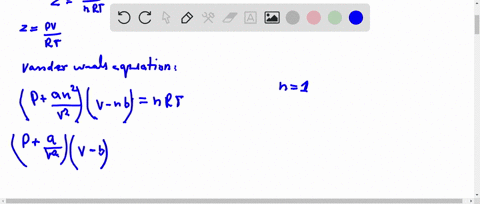
If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
$ 8.50 · 4.7 (357) · In stock

The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
For one mole of a van der Waals gas when b0andT300K the PVvs1Vplot is shown below The value of the van der Waals constant aatmL2mol2is

⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…

20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Which of these are correct? A) Z, compressibility factor, low pressure can be written as z = B) Z, low pressure can be written as z = 1 + P C) Z

If Z is a compressibility factor, van der Waals equation at low pressure ..
⏩SOLVED:If Z is a compressibility factor, van der Waals equation at…

JEE Mains, Chemistry, Study Material
Class Xi States of Matter, PDF, Gases

SOLVED: I need the answer as soon as possible. 16. If Z is a compressibility factor, van der Waals' equation at low pressure can be written as: (P + (an^2/V^2))(V - nb) =

66. If z is the compressibility factor, van der Waals equation low pressure can be written as: (A) Z = 1 + PT (B) 2 = 1 - VT (C) 2=1 - (0) 2 =1+ PT Space rough use
Solved papers for JEE Main & Advanced JEE Main Solved Paper-2014
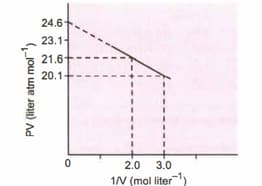
For one mole of a van der Waals gas when b0andT300K the PVvs1Vplot is shown below The value of the van der Waals constant aatmL2mol2is
