In the following compressibility factor (Z) vs. pressure graph 300
$ 14.00 · 5 (574) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Adoption of machine learning in estimating compressibility factor for natural gas mixtures under high temperature and pressure applications - ScienceDirect

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

Energies, Free Full-Text

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Compressibility factor - Wikipedia

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange
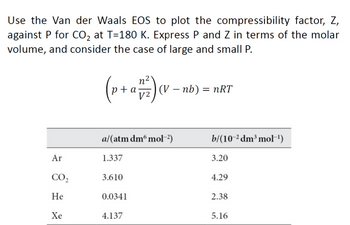
Answered: Use the Van der Waals EOS to plot the…

Compressibility Charts McGraw-Hill Education - Access Engineering