Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
$ 14.50 · 4.6 (362) · In stock


Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

1. The compressibility factor, z, is the ratio of
Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor Z - Gaseous State

Properties of gases extended oct 2020

Real Gas Behavior The Compression Factor (Z) [Example #2]

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

PPT - We now turn our attention to the concept of pure substances and the presentation of their data. PowerPoint Presentation - ID:4757289
Gas compressibility factor Z: Ideal gas vs Real gas
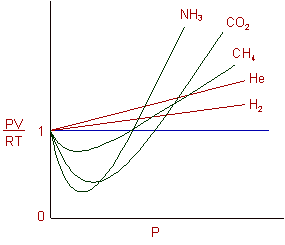
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
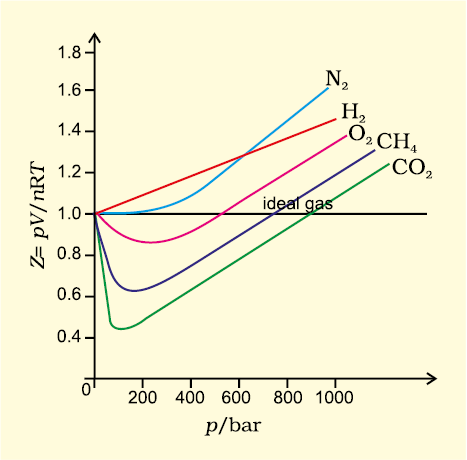
Sections

3.3: Real gas and compressibility factor - Engineering LibreTexts

Chemistry!!! Not Mystery : Do Real Gases Behave Ideally?

