The FDA's rule change requiring providers to inform women about
$ 17.00 · 4.8 (212) · In stock

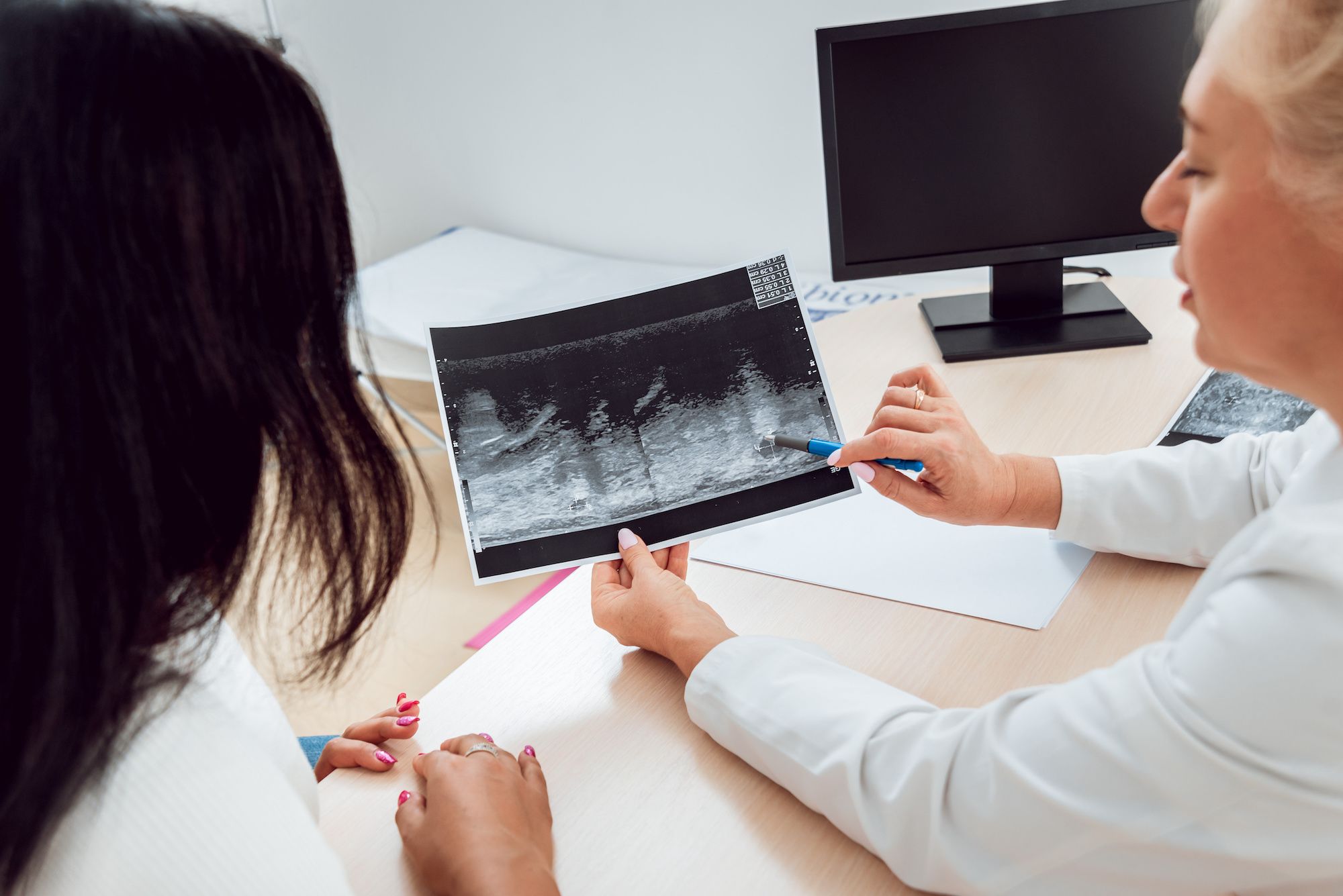
FDA to require mammogram reports include breast density information

FDA proposes new regulations to increase oversight of Laboratory Developed Tests

Vital Signs: Digital Health Law Update, Fall 2023, Insights
Women's Healthcare, A Clinical Journal for NPs on LinkedIn: The

It's not how much we've done, but how much we are doing!' - UPMC

FDA Will Require Dense Breast Disclosure at Mammogram Clinics - The New York Times

FDA to require mammogram providers to notify women about breast density to help detect breast cancer sooner

What Are Dense Breasts? FDA Requires Information In Mammogram Results

The FDA's rule change requiring providers to inform women about

The FDA's rule change requiring providers to inform women about
