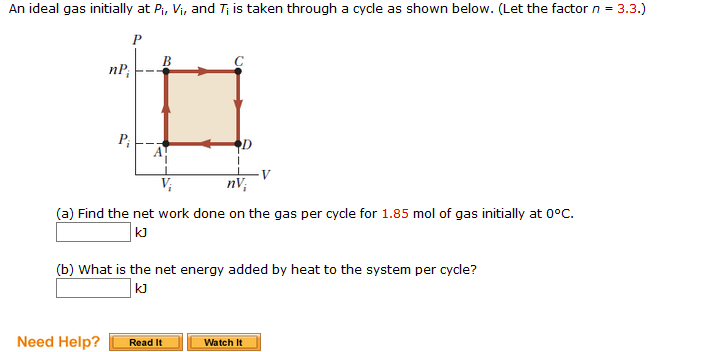
Solved An ideal gas initially at Pi, Vi, and Ti is taken
$ 32.00 · 4.8 (663) · In stock

1 mole of an ideal gas at initial temperature of T K does 6 R joules of work adiabatically. If the ratio of specific heats of this gas at constant pressure and
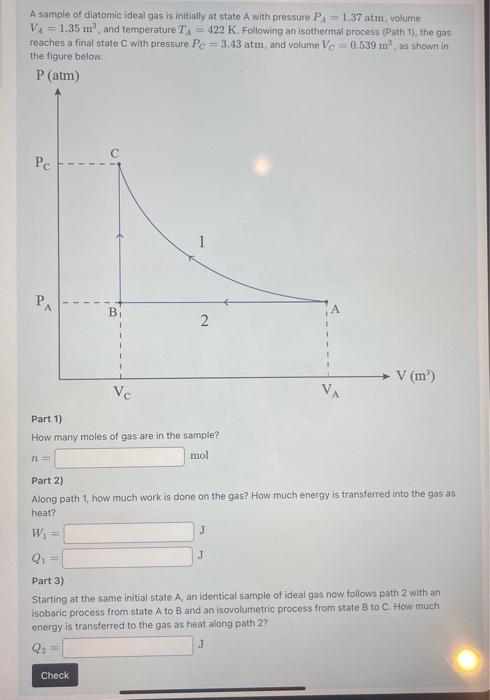
Solved A sample of diatomic ideal gas is initially at state
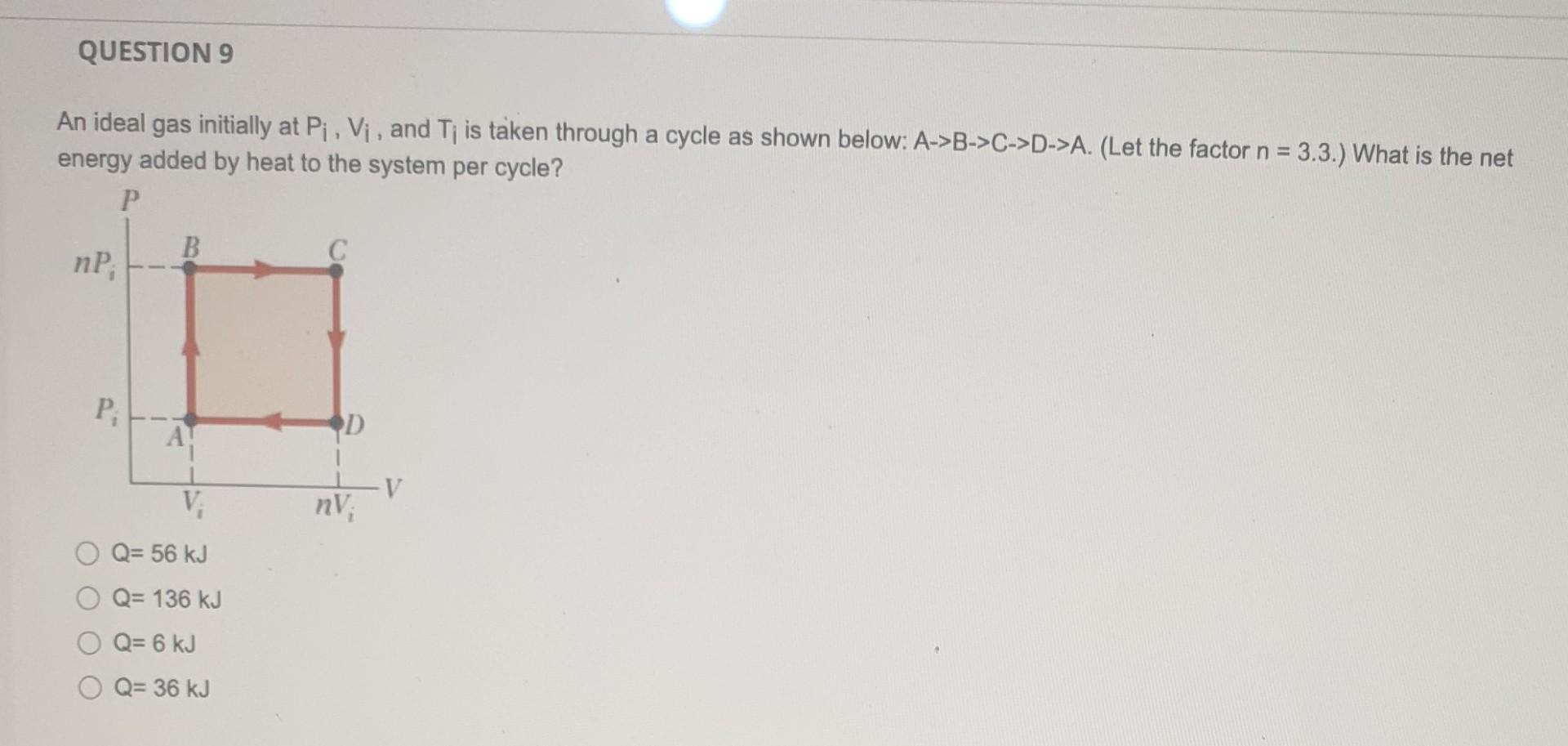
Solved An ideal gas initially at Pi,Vi, and Ti is taken
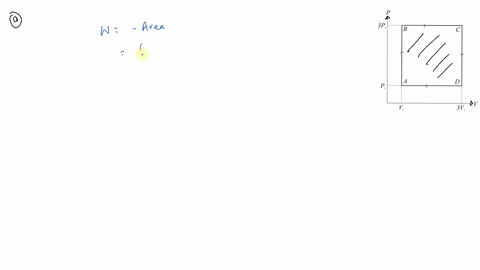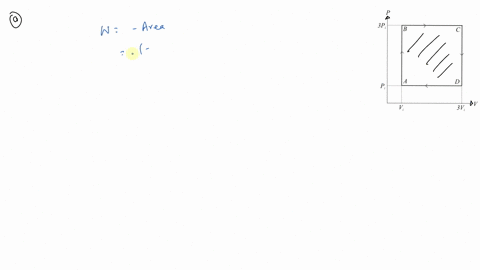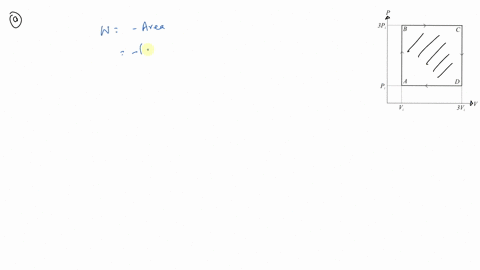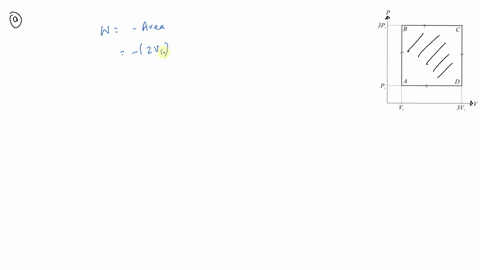
⏩SOLVED:An ideal gas initially at Pi, Vi and Ti is taken through a…

⏩SOLVED:An ideal gas initially at Pi, Vi and Ti is taken through a…
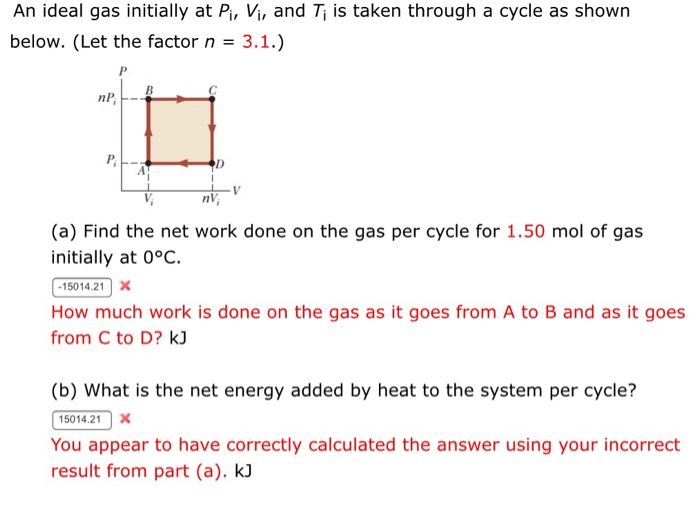
Solved An ideal gas initially at Pi, Vi, and Ti is taken
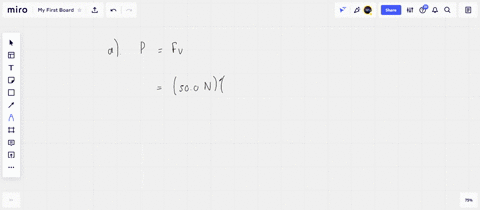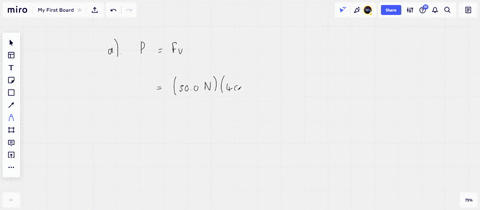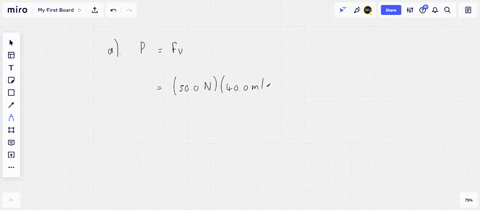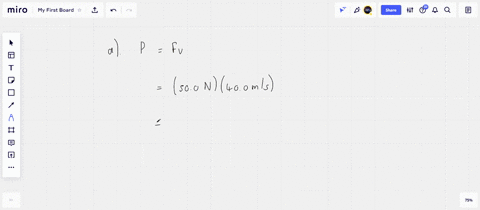
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

Solved An ideal gas initially at Pi, Vi, and T is taken
The ideal gas law (PV = nRT) (video)

Keyhole fluctuation and pore formation mechanisms during laser powder bed fusion additive manufacturing

mohol an Ideal gas al 300 K occupies a volume of 0.36 m of 2 atm. The gas expands adiabatically its volume becomes 144. Net gas is compressed isobarically to its original

⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure (Pressure in Pa and Volume in m^3) What is the net energy