the compression factor one mole of a vander waals gas 0 C and 100
$ 24.99 · 5 (113) · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor for one mole of a vander waals gas at 0 c and
Click here👆to get an answer to your question ✍️ The compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0-5

The compression factor (compressibility factor) for 1 mol of a van der

Djective lype Questions The compressibility factor of N2 moderate pressure range is equal to [where a & b are the van der Waal's constants] Pb Pb (2) 1- (1)RT RTV (3) (1RTV

2 mol of ammonia occupied a volume of 5 L at 27∘ C. Calculate the pressure if the gas obeyed van der Waals equation. a = .4.17 atm L 2 mol 2

Density of van der Waals' gas 500 K and 1.0 atm was found to be 0.8 kg/m'. Also gas was found to effuse 1.37 times slower than oxygen under identical condition. Determine

Solved (Triple-Play Bonus) For a certain gas, the
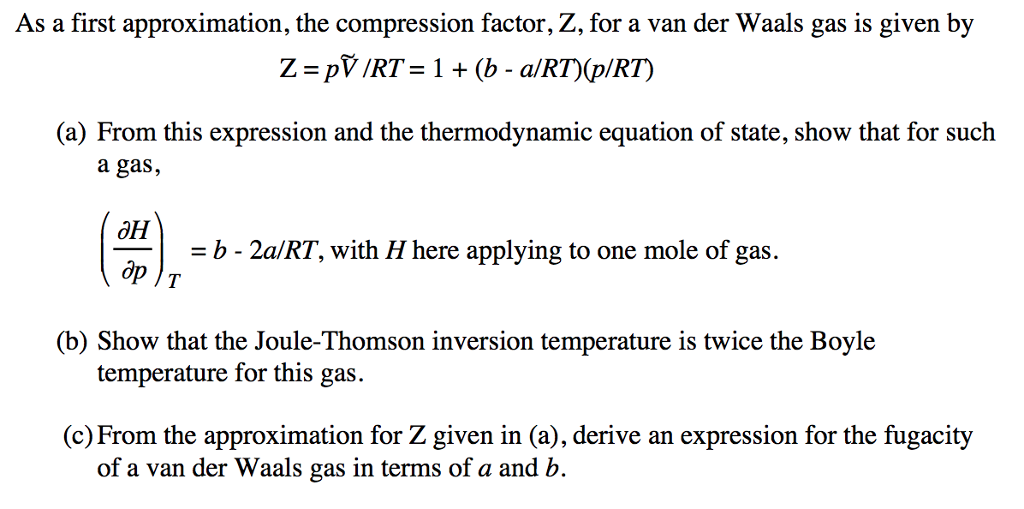
As a first approximation, the compression factor, Z
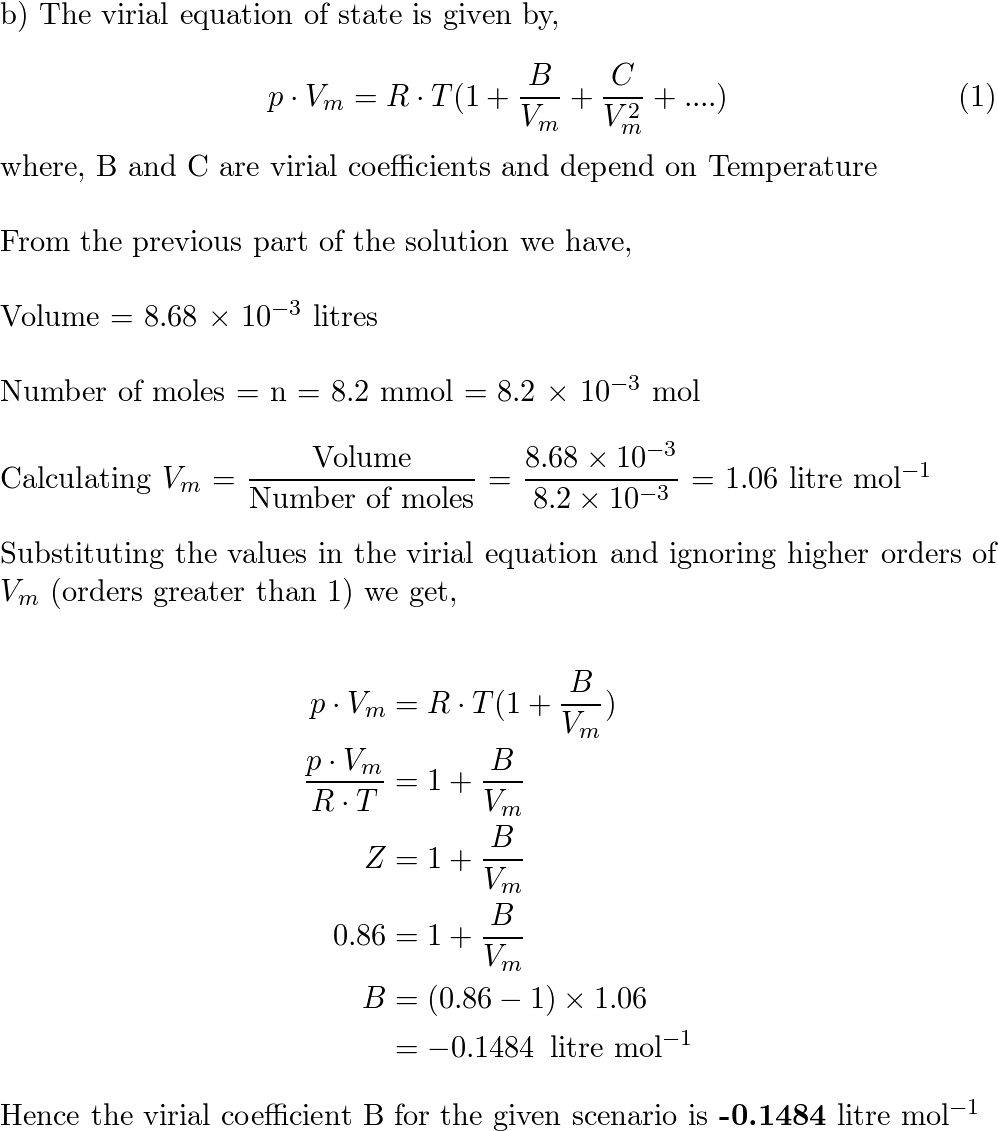

The compression factor (compressibility factor) for one mole of a van der..

The compression factor (compressibility factor) for one mole of a Van der..
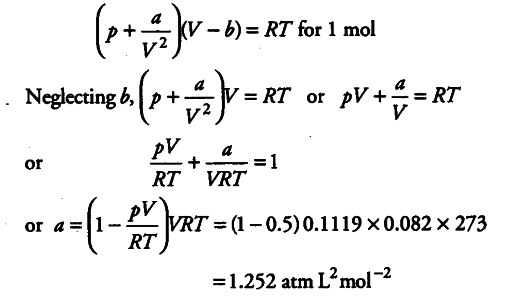
The compression factor (compressibility factor) for one mole of a van - CBSE Class 11 Chemistry - Learn CBSE Forum
How to calculate the values of critical pressure and temperature for a given gas (Van der Waals equation) - Quora

Calculate the molar volume of argon at 100c and 100 atm on the assumption that it is a van der Waals

The compression factor compressibility factor for 1 mole of a van der Waals' gas at 0∘ C and 100 atmospheric pressure is found to be 0.5 . Assuming that the volume of