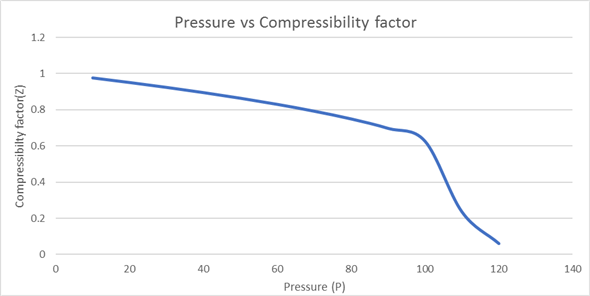
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
$ 22.99 · 5 (352) · In stock

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state
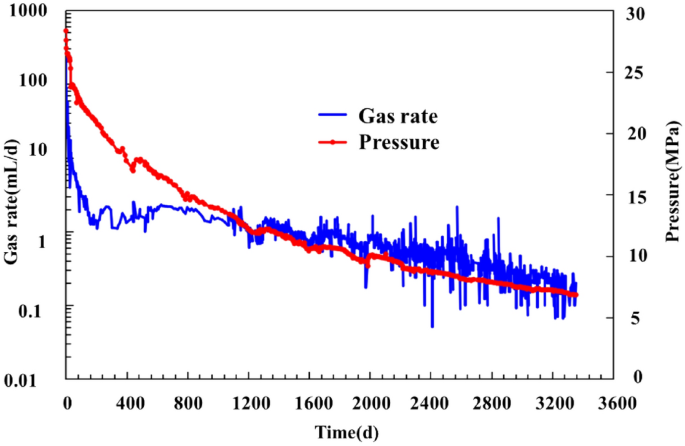
Experimental and numerical study on gas production decline trend under ultralong-production-cycle from shale gas wells
Solved) - Equation (3.34) when truncated to four terms accurately represents (1 Answer)
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora
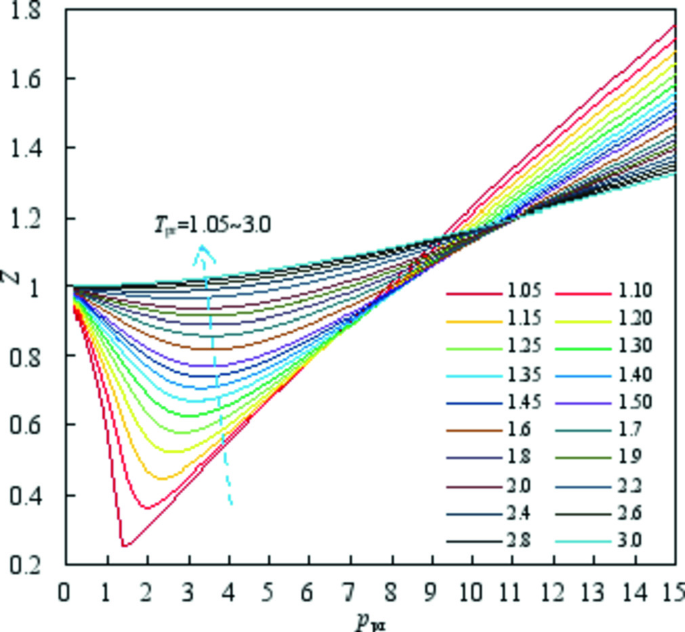
A New Method for Estimating Compressibility Factors of Natural Gases Based on Bender Equation of State

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download
At which pressure methane gas becomes non ideal? - Quora

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download

The graph of compressibility factor (Z) vs. P for one mole of a

Thermodynamics: An Engineering Approach - 5th Edition - Part II by 黑傑克 - Issuu