The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT - Sarthaks eConnect
$ 6.50 · 4.9 (709) · In stock
The compressibility factor for a real gas at high pressure is (a) 1+RT/pb (b) 1 (c) 1+pb/RT (d) 1-pb/RT

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
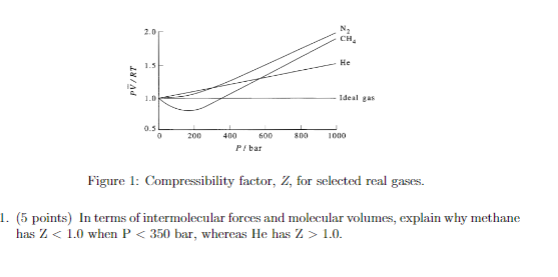
Solved CH 1.5 He PV/RT 1.0 Ideal gas 0.5 200 800 1000 400

Simple Equation Real Gas Compressibility Factor Z

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
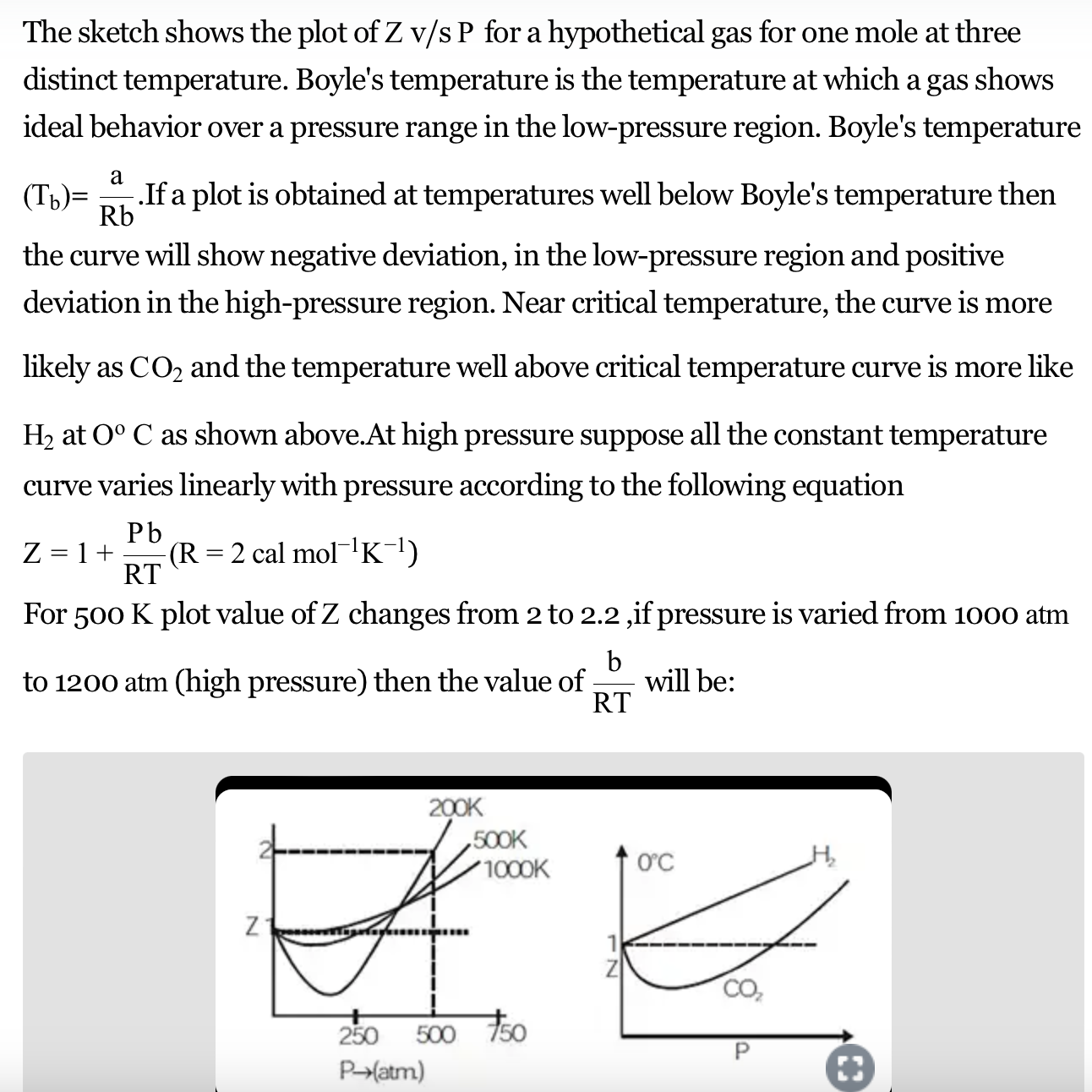
Solved The graph of compressibility factor (Z)v/sP for 1 mol

1. The compressibility factor, z, is the ratio of
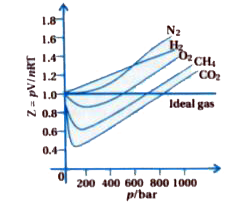
Gujrati] Explain compressibility factor (Z).

The compressibility factor a real gas high pressure is RT (b)1 po

gas laws - Graph of compressibility factor vs pressure when real

States Of Matter Notes: Class 11, JEE, NEET, AIIMS
Solved The graph of compressibility factor (Z)v/sP for 1 mol

The compressibility factor of a gas is defined as Z=PV/nRT. The