Postembryonic RNAi in Heterorhabditis bacteriophora: a nematode insect parasite and host for insect pathogenic symbionts, BMC Developmental Biology
$ 28.50 · 4.8 (129) · In stock
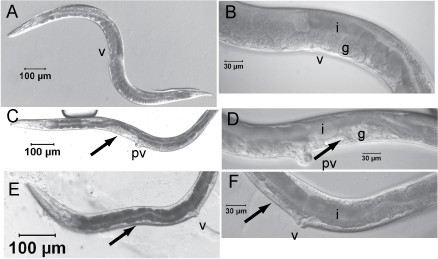
Background Heterorhabditis bacteriophora is applied throughout the world for the biological control of insects and is an animal model to study interspecies interactions, e.g. mutualism, parasitism and vector-borne disease. H. bacteriophora nematodes are mutually associated with the insect pathogen, Photorhabdus luminescens. The developmentally arrested infective juvenile (IJ) stage nematode (vector) specifically transmits Photorhabdus luminescens bacteria (pathogen) in its gut mucosa to the haemocoel of insects (host). The nematode vector and pathogen alone are not known to cause insect disease. RNA interference is an excellent reverse genetic tool to study gene function in C. elegans, and it would be useful in H. bacteriophora to exploit the H. bacteriophora genome project, currently in progress. Results Soaking L1 stage H. bacteriophora with seven dsRNAs of genes whose C. elegans orthologs had severe RNAi phenotypes resulted in highly penetrant and obvious developmental and reproductive abnormalities. The efficacy of postembryonic double strand RNA interference (RNAi) was evident by abnormal gonad morphology and sterility of adult H. bacteriophora and C. elegans presumable due to defects in germ cell proliferation and gonad development. The penetrance of RNAi phenotypes in H. bacteriophora was high for five genes (87–100%; Hba-cct-2, Hba-daf-21, Hba-icd-1; Hba-nol-5, and Hba-W01G7.3) and moderate for two genes (usually 30–50%; Hba-rack-1 and Hba-arf-1). RNAi of three additional C. elegans orthologs for which RNAi phenotypes were not previously detected in C. elegans, also did not result in any apparent phenotypes in H. bacteriophora. Specific and severe reduction in transcript levels in RNAi treated L1s was determined by quantitative real-time RT-PCR. These results suggest that postembryonic RNAi by soaking is potent and specific. Conclusion Although RNAi is conserved in animals and plants, RNAi using long dsRNA is not. These results demonstrate that RNAi can be used effectively in H. bacteriophora and can be applied for analyses of nematode genes involved in symbiosis and parasitism. It is likely that RNAi will be an important tool for functional genomics utilizing the high quality draft H. bacteriophora genome sequence.

Nematode Information — Nematode Information
RNA Processing, PDF, Rna Splicing

RNAi-mediated gene knockdown by microinjection in the model entomopathogenic nematode Heterorhabditis bacteriophora, Parasites & Vectors
Silencing of Aphid Genes by dsRNA Feeding from Plants

PDF) RNAi-mediated gene knockdown by microinjection in the model entomopathogenic nematode Heterorhabditis bacteriophora

Systemic RNAi mediated gene silencing in the anhydrobiotic nematode Panagrolaimus superbus. - Abstract - Europe PMC
A Lover and a Fighter: The Genome Sequence of an Entomopathogenic Nematode Heterorhabditis bacteriophora
Insect Immunity to Entomopathogenic Nematodes and Their Mutualistic Bacteria
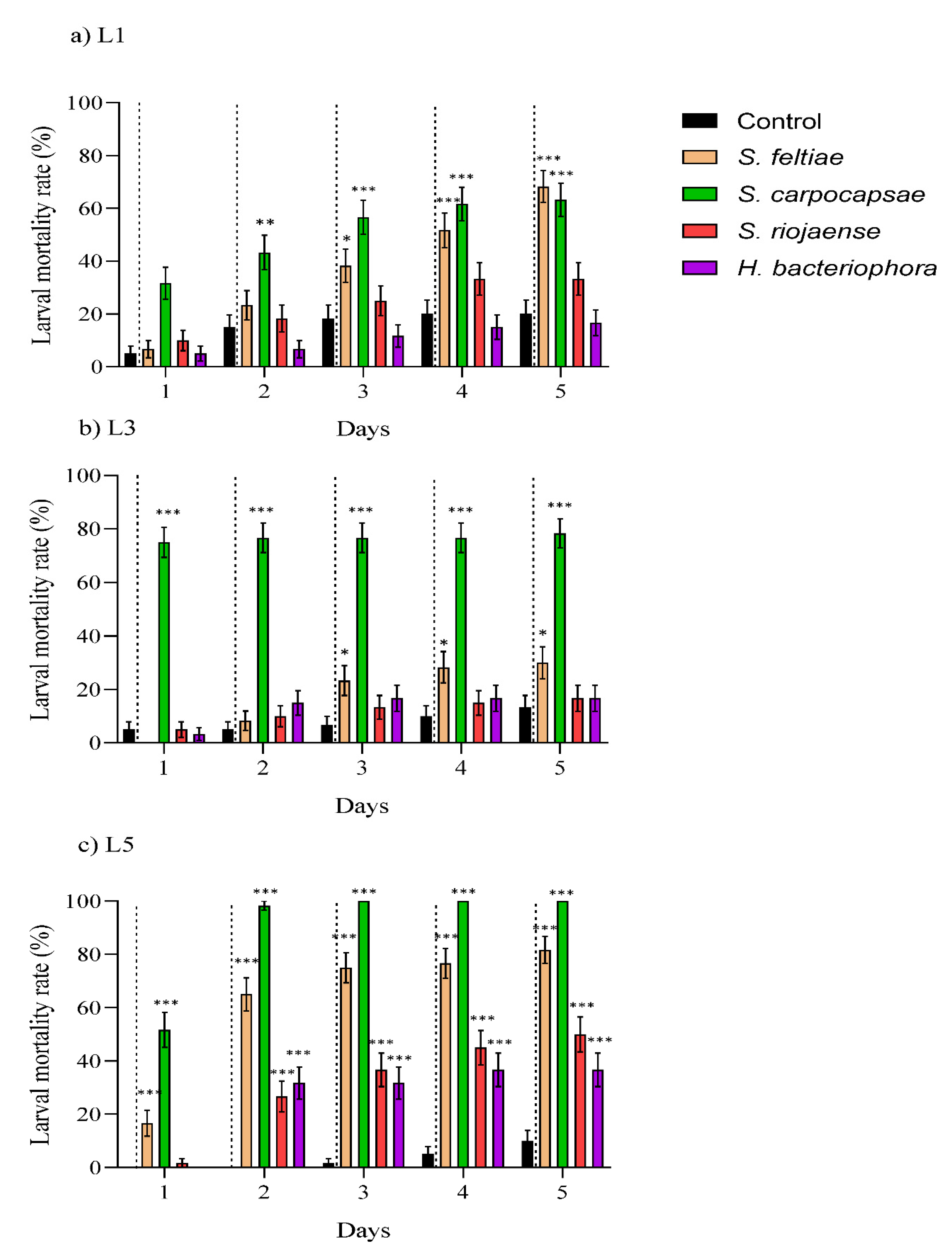
Insects, Free Full-Text
A Lover and a Fighter: The Genome Sequence of an Entomopathogenic Nematode Heterorhabditis bacteriophora

Molecular Microbiology, Microbiology Journal

Several Grassland Soil Nematode Species Are Insensitive to RNA-Mediated Interference. - Abstract - Europe PMC

A neuropeptide modulates sensory perception in the
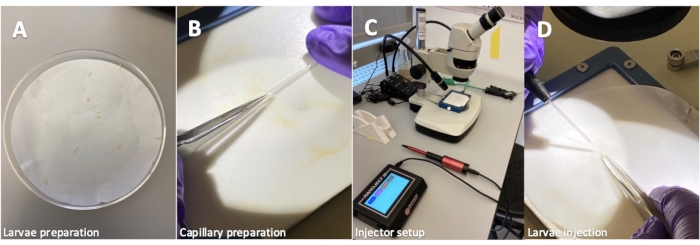
Drosophila melanogaster Larva Injection Protocol

Frontiers Nematophilic bacteria associated with entomopathogenic nematodes and drug development of their biomolecules

