Solace initiates enrolment in trial of bladder control balloon
$ 17.50 · 4.9 (371) · In stock

Solace’ WomanCare intiated enrolement in VESAIR Clinical Trial to test safety and efficacy of Vesair Bladder Control Balloon procedure.

Design and rationale of a prospective, randomized, non-inferiority trial to determine the safety and efficacy of the Biolimus A9™ drug coated balloon for the treatment of in-stent restenosis: First-in-man trial (REFORM)

DevEng – Blum Center

TCWN February 4 - 10, 2023 by TC Weekly News - Issuu

Breast Cancer Awareness Month

Concerned United Birthparents (CUB) Natl ~ Pg 303-424 - triadoption

CardiovaScUlar toxicity in cancer and improvement In recovery

BayouLife Magazine August 23 by BayouLife Magazine - Issuu
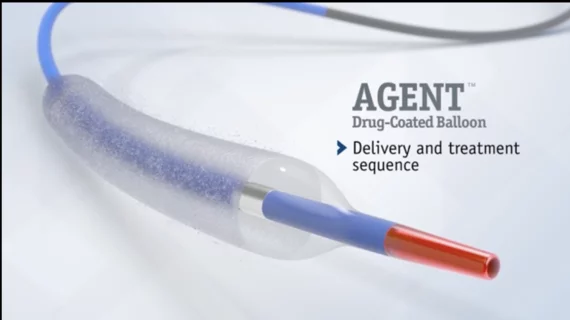
FDA approves Boston Scientific's new drug-coated balloon for coronary in-stent restenosis

Aprils have never meant much to me, autumns seem that season of beginning, spring - ART FLAIR

North Carolina Literary Review Online Winter 2024 by East Carolina University - Issuu
M SpringerLink

Blockchain Burger #12 - Blockchain Burger Factory

Minimal Device Encrustation on Vesair Intravesical Balloons in the Treatment of Stress Urinary Incontinence: Analysis of Balloons Removed from Women in the SOLECT Trial

Solace Therapeutics Announces Enrollment of First Patient in the SUCCESS Pivotal Trial to Assess Safety and Effectiveness of the Solace Bladder Control Balloon System™ for the treatment of Stress Urinary Incontinence (SUI) in Women.

Minimal Device Encrustation on Vesair Intravesical Balloons in the Treatment of Stress Urinary Incontinence: Analysis of Balloons Removed from Women in the SOLECT Trial
