Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
$ 24.50 · 4.5 (397) · In stock

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Arihant Chemistry Sample Paper Class 11 by KnowledgeTest - Issuu

gaseous state
The Behavior of Gases Chemistry for Non-Majors

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Production Engineering - ScienceDirect
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora
Compressibility factor Z is plotted against pressure p for four different gases A , B , C & D. The correct order of critical temperature of the gasesA. A>B>C>DB. B>A>C>DC. D
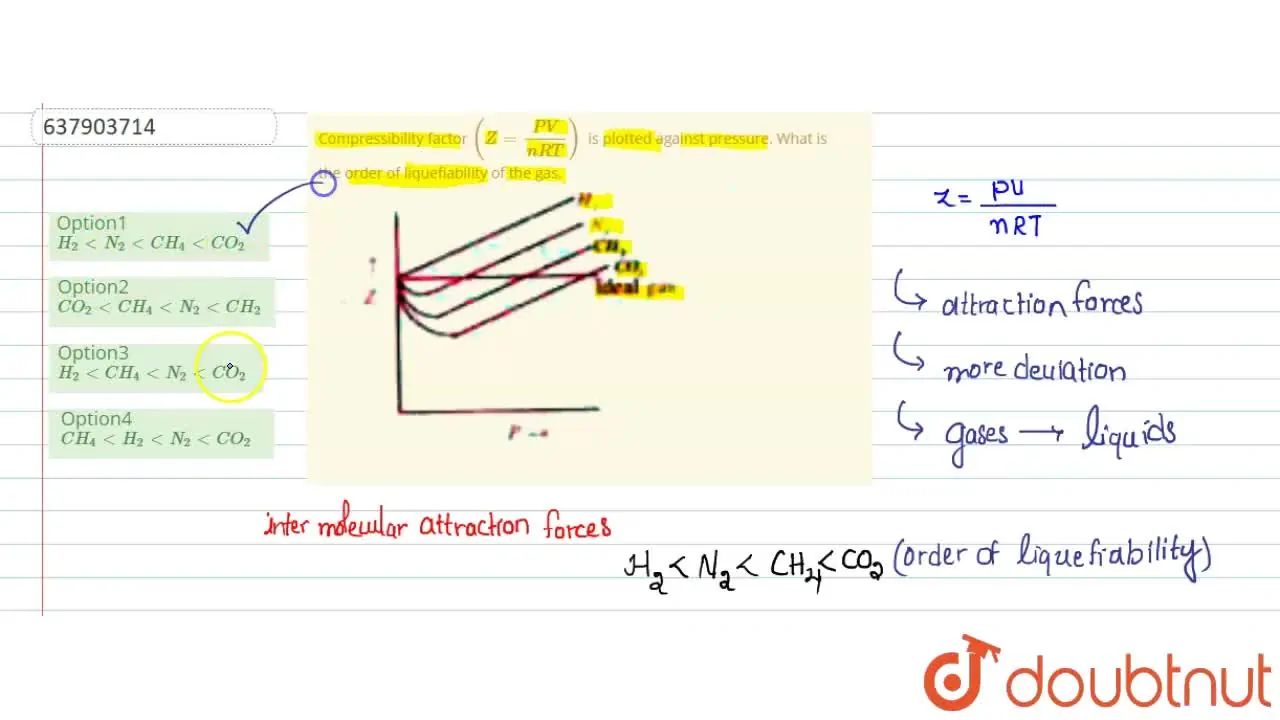
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p

Sustainability June-2 2023 - Browse Articles

CO2 Z =1 What is the correct increasing order of liquifiability of
