Solved] Why is the compressibility factor less than 1 at most
$ 19.99 · 4.7 (167) · In stock
Answer to Why is the compressibility factor less than 1 at most conditions?

Compressibility factor (z): real gases deviate from ideal behav-Turito

Solved The compressibility factor, Z, can be thought of as a

What is compressibility factor? What is its value for ideal gas

Compressibility factor - Wikipedia

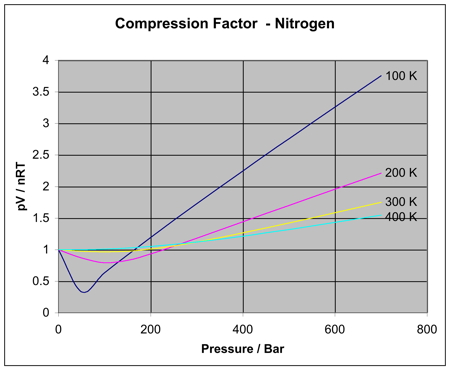
Reading Compressibility Factor Charts
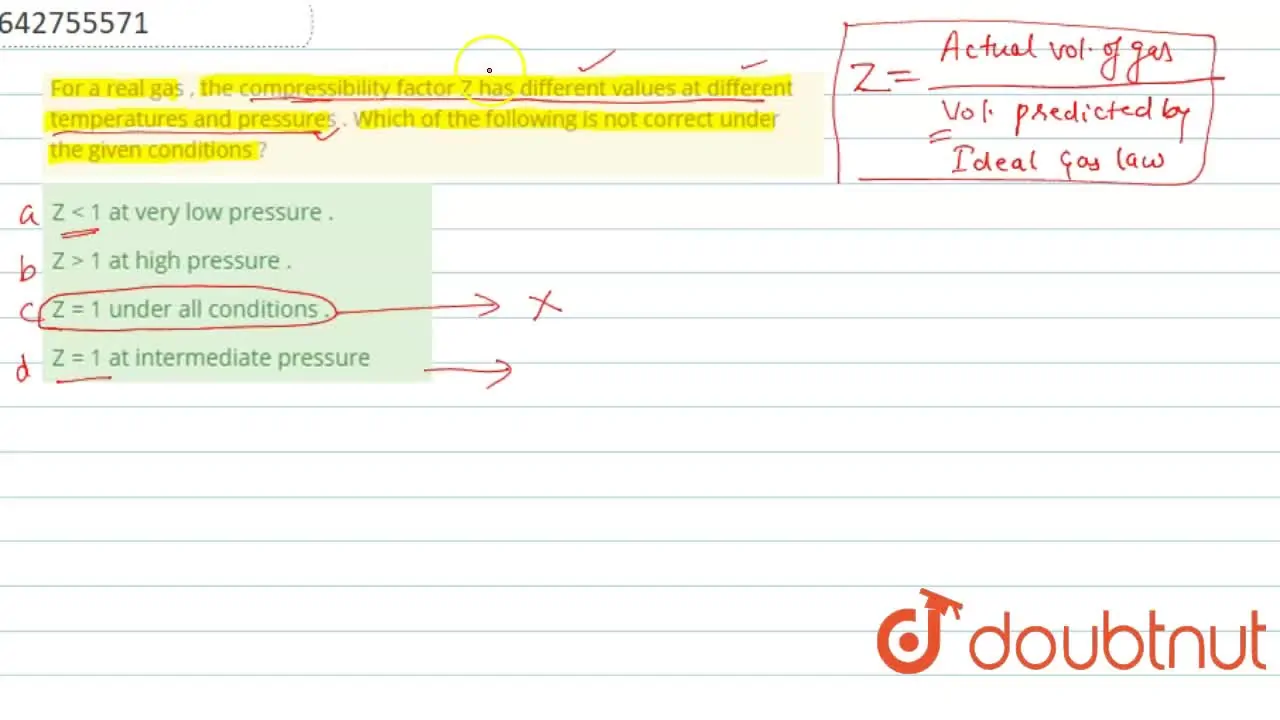
For a real gas , the compressibility factor Z has different values at
Non-ideal behavior of gases (article)

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (gases) - Knowino

Miscellaneous Contributions by: - ppt video online download
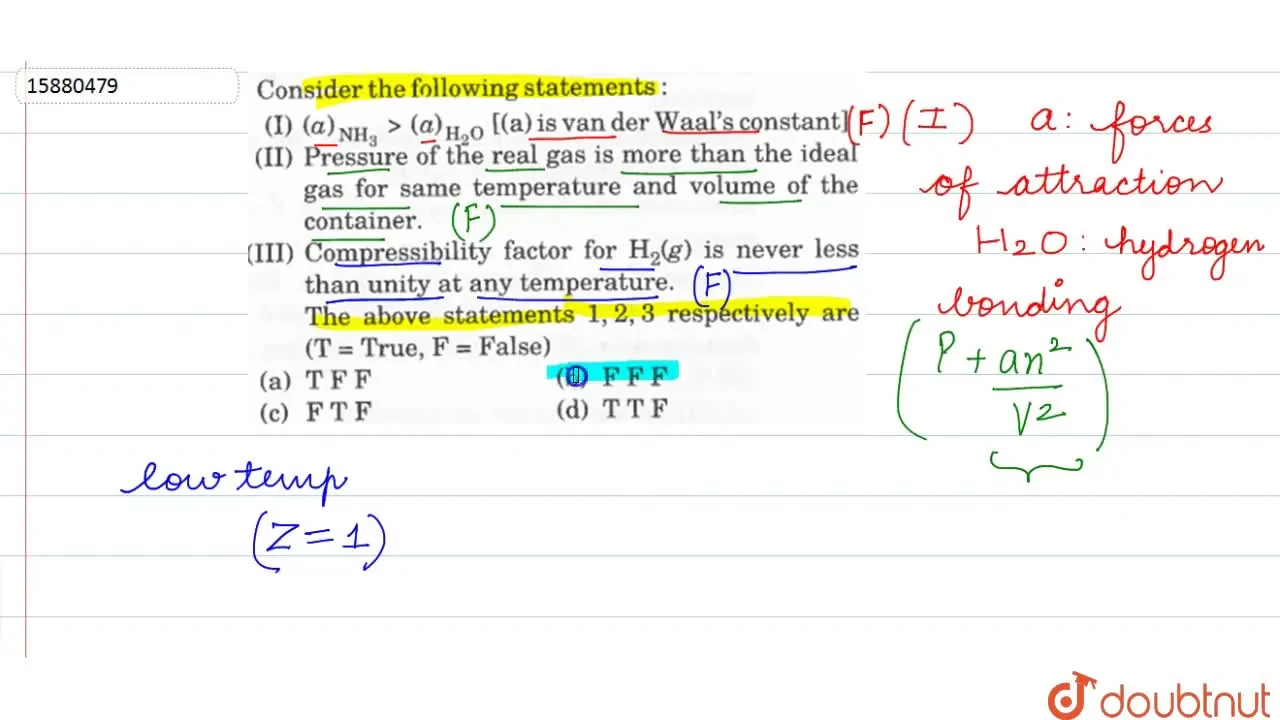
Consider the following statements: (I) (a)(NH(3))gt(a)+(H(2)O) [(a)
Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility Factor - an overview

Van der Waals Equation - Derivation, Relation Between Ideal Gas
