What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
$ 13.00 · 4.9 (759) · In stock
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas

At high pressure, the compressibility factor for one mole of van der w

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

Compression ratio, ρ2/ρ1, and P rad /P th for hydrogen, considered as a
Solved 9 Compression factor Z Use the van-der-Waals equation

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Solved Problem 2. ( 30 points) The ideal gas law can

SOLVED: I need the answer quickly. Hi-condition toop. I want a solution in Matlab. Write a computer program to calculate molar volume and compressibility factor for gaseous ammonia by using the Redlich-Kwong

If Z is a compressibility factor, van der Waals equation at low pressure ..
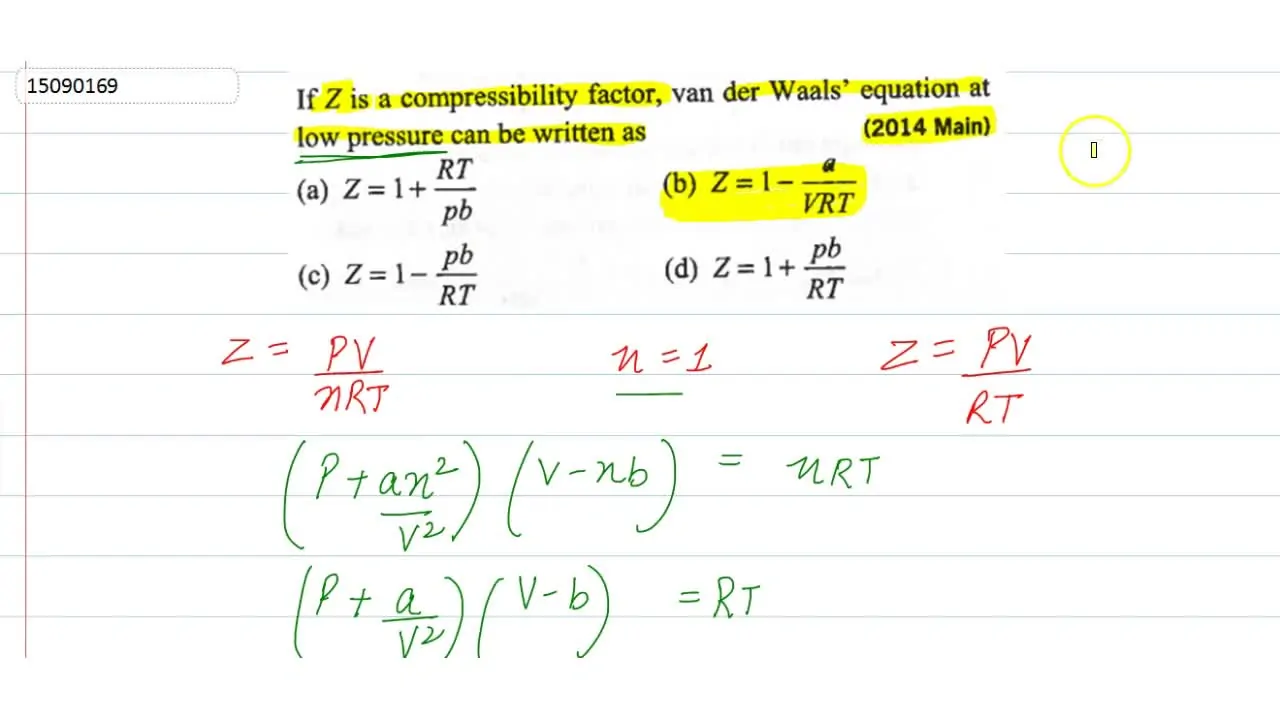
If Z is a compressibility factor, van der Waals' equation at low press

Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

16.4: The Law of Corresponding States - Chemistry LibreTexts
