OneClass: For a real gas, the compressibility factor, Z, is
$ 9.50 · 4.5 (304) · In stock


Compressibility factor (gases) - Knowino

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

What is compressibility factor? What is its value for ideal gas

Energies, Free Full-Text

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z =(1-displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})
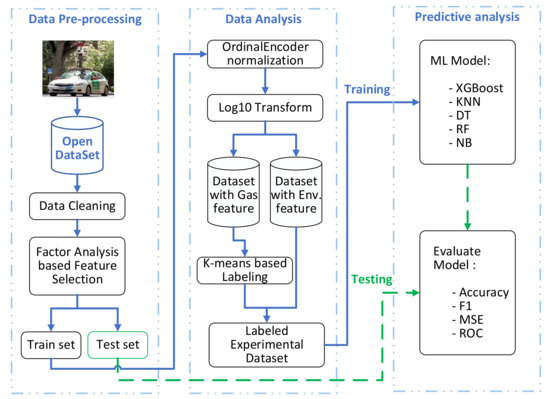
Applied Sciences, Free Full-Text

OneClass: #1 The Joule-Thomson inversion locus is the set of points for which the Joule-Thomson coeff
States of Matter Class 11 Notes CBSE Chemistry Chapter 5 [PDF]
Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia

Solved Real gas effects can be expressed as departures from

Compressibility factor for real gases
