Solved] Why is the compressibility factor less than 1 at most conditions?
$ 27.50 · 4.6 (263) · In stock

Real Gases Introductory Chemistry

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Non-Ideal Gas Behavior Chemistry: Atoms First
Solved] Why is the compressibility factor less than 1 at most conditions?
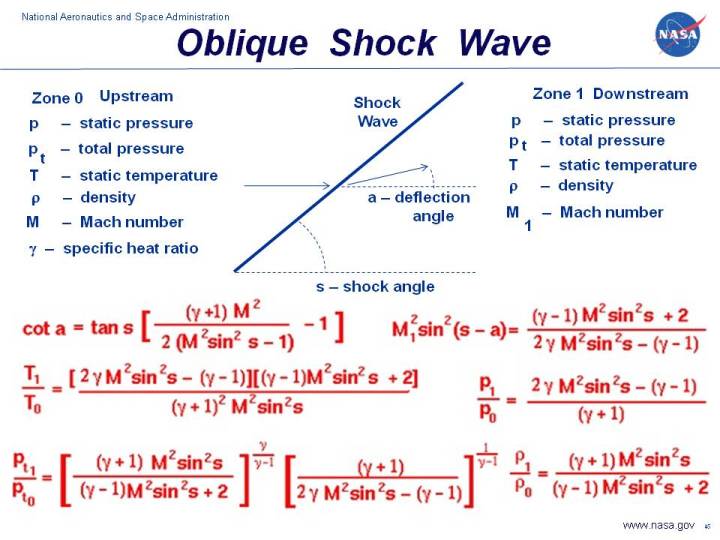
Oblique Shock Waves

The compressibility of a gas is less than unity at STP. Therefore, its molar volume is

Compressibility Factor Z Important Concepts and Tips for JEE Main

Non-Ideal Gas Behavior Chemistry: Atoms First
The compressibility of a gas is than unity STP, therefore:V_{m} > 22.4 LV_{m} < 22.4 LV_{m} = 22.4 LV_{m} = 44.8 L

Compressibility Factor - an overview

SOLUTION: Statistical molecularthermodynamics homework solution2 - Studypool

Physical Chemistry The Compression Factor (Z) [w/1 example]
The value of compression factor at the critical state of a vander waals gas is

Compressibility factor - Wikipedia

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
