For each of the negative ions listed in column 1, use the periodic table to find in column 2 the total number of electrons the ion contains. A given answer may be
$ 8.99 · 4.9 (225) · In stock

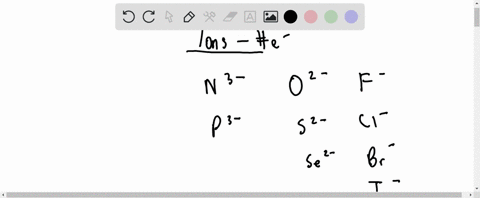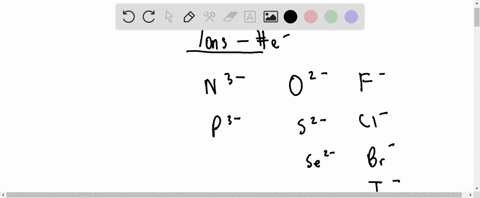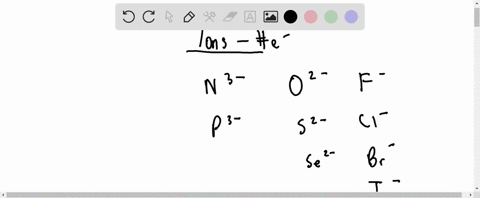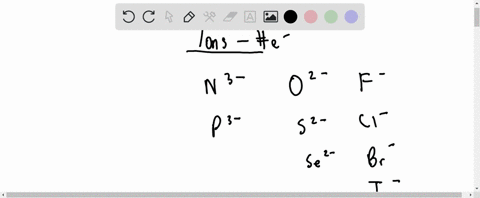
⏩SOLVED:For each of the negative ions listed in column 1, use the…
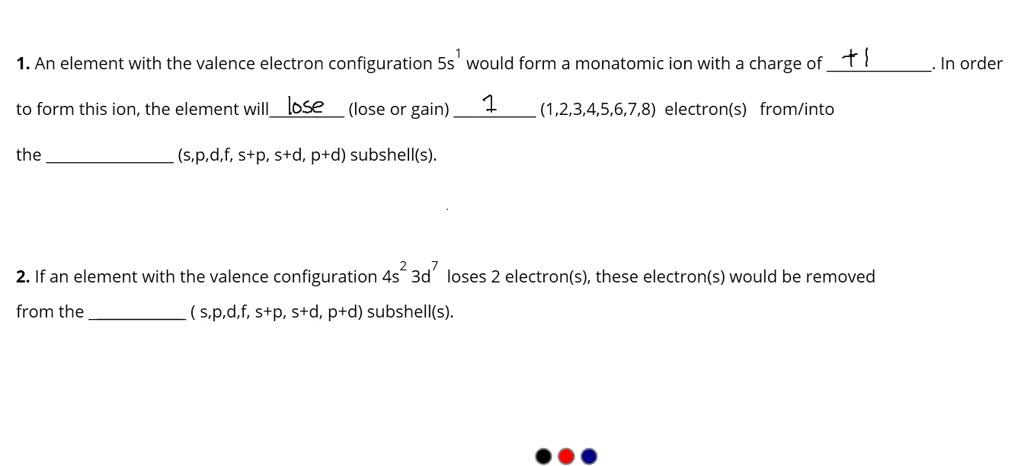
SOLVED: An element with the valence electron configuration 5s1 would form a monatomic ion with a charge of . In order to form this ion, the element will (lose or gain) (1

Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
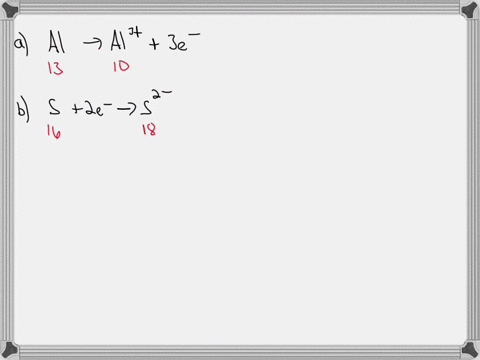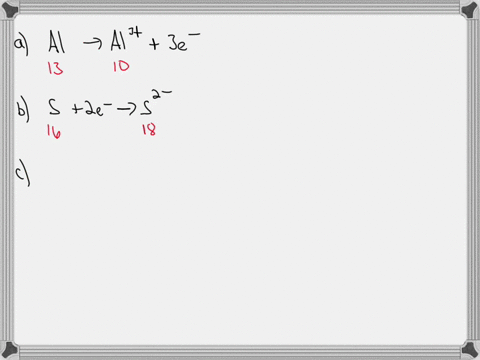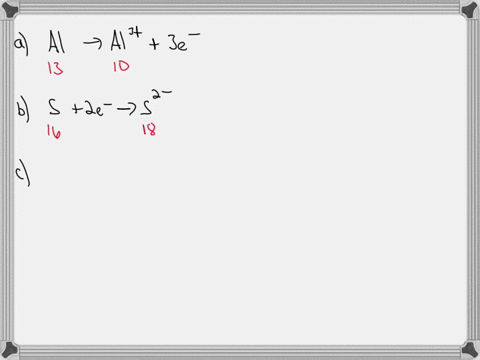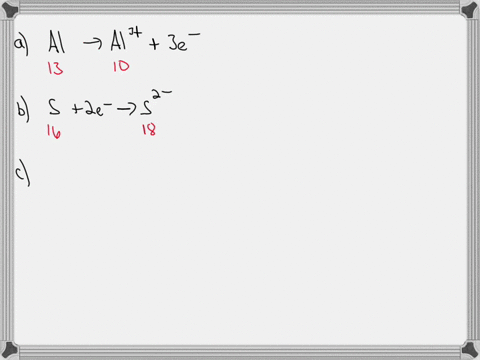
SOLVED: An ion is formed when an atom gains or loses an electron or electrons. Ions have a charge. If an atom has seven electrons in the outer shell, it will tend
Fill in the blanks to complete the table., Symbol, Ion Comm
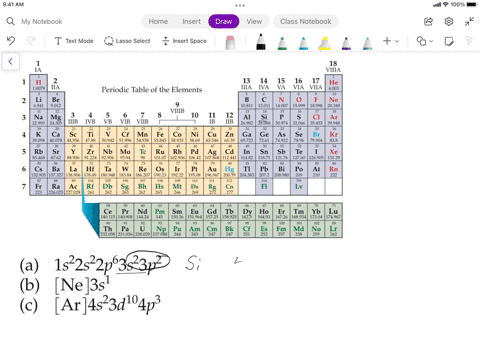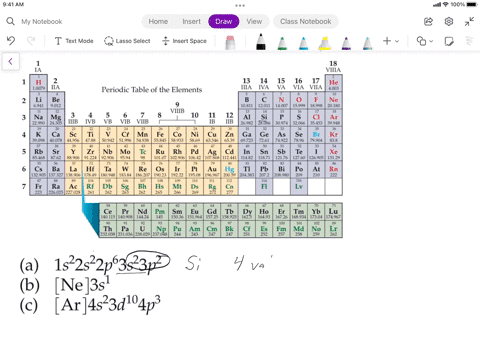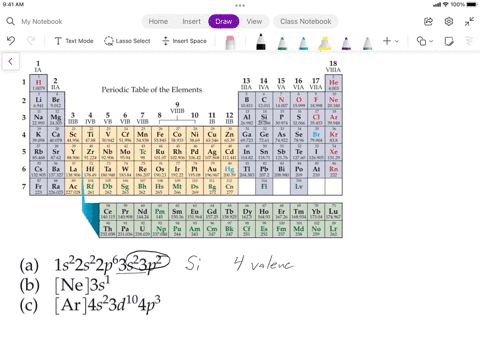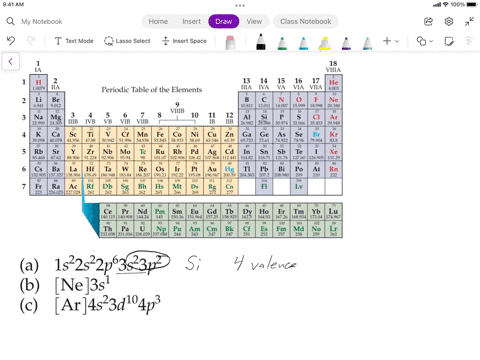
SOLVED: Assuming the metals lose all their valence electrons, and the nonmetals gain electrons to complete the s-p subshells, which listing below shows the correct information for the element? Elements Electron Structure

Chem Unit 3 Ions Answers - Standards: 3.1.10 B Describe concepts of models as a way to predict and understand science and technology. 3.4.10 A Explain

SOLVED: Fill in the blanks to complete the following table. Symbol Ion Commonly Formed Number of Electrons in Ion Number of Protons in Ion F F- 10 9 Be2+ Be2+ 2 4

Chem Unit 3 Ions Answers - Standards: 3.1.10 B Describe concepts of models as a way to predict and understand science and technology. 3.4.10 A Explain
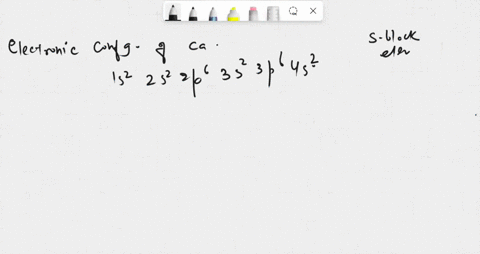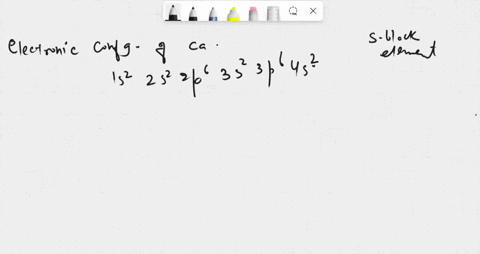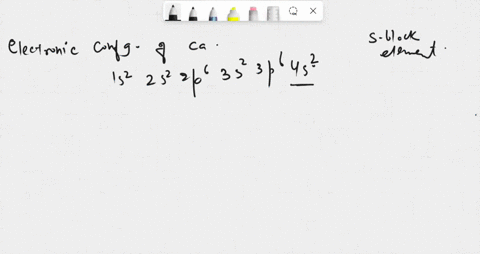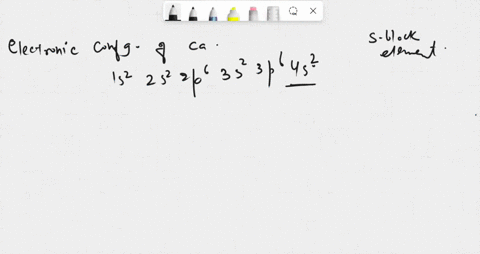
SOLVED: in many compounds, atoms of main-group elements form ions so that the number in the outermost energy levels of each ion is

SOLVED: Assuming the metals lose all their valence electrons, and the nonmetals gain electrons to complete the s-p subshells, which listing below shows the correct information for the element? Elements Electron Structure

SOLVED: Question 13: Give the number of electrons for the following ions: Mass number: 52 24Cr+3 80 3SBr A. Cr: 55; Br: 79 B. Cr: 49; Br: 81 C. Cr: 21; Br: 36 D. Cr: 28; Br: 45 E. Cr: 27; Br: 34

SOLVED: in many compounds, atoms of main-group elements form ions so that the number in the outermost energy levels of each ion is

Atomic Structure d. Atomic Structure d Atomic Structure d Electron (negative) Neutron (neutral) Proton (positive) d nucleus. - ppt download

SOLVED: For each of the positive ions listed in column 1 use the periodic table to find in column 2 the total number of electrons that ion contains. The same answer may